Abstract
Research Article
Determination of the in vivo activity of leaves extract of Zanthoxylum Chiloperone var. Angustifolium (Tembetary hú) orally and intralesionally administered to BALB/c mice experimentally infected with Leishmania
Elva Serna*, Marisel Maldonado and Nilsa González
Published: 23 November, 2022 | Volume 6 - Issue 1 | Pages: 038-043
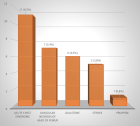
Natural products are becoming increasingly important as an unlimited source for obtaining chemical substances with possible pharmacological potential. Current existing drugs for the treatment of cutaneous leishmaniosis produce major side effects; therefore the search for new drugs is justified. The stem bark of Zanthoxylum chiloperone var. Angustifolium Engl. (Rutaceae) is traditionally used in Paraguay for its antiparasitic properties. The leaf extract was evaluated for the first time to determine its leishmanicidal activity in BALB/c mice infected with amastigote forms of Leishmania amazonensis (PH8). The mice were treated orally with the extract at three concentrations (100, 50 and 10 mg/mL), intralesional (50 mg/mL), and subcutaneously using glucantime as a control (100 mg/mL). The percentage of decrease in parasite load was measured and with intralesional 50 mg/kg a reduction of 72% occurred, with the reference drug (Glucantime) a reduction of 62% was obtained with the same oral dose a reduction of 50%, while with an oral dose of 10 mg/mL the percentage of reduction was 55%. When the oral dose was increased to 100 mg/mL, the reduction percentage of the parasitic load was only 16%. These results indicated that the leaf extract of Z. chiloperone var. angustifolium Engl. at low oral concentrations (50 and 10 mg/mL) had very good activity against L. amazonensis, and it was even more efficacious intralesionally at 50 mg/mL but at the oral dose of 100 mg/kg has very reduced antiparasitic activity. This study showed the efficacy of the extract leaves of Z. chiloperone in reducing the parasite load in an in vivo test, so its use as a potential leishmanicidal could be suggested to develop and evaluate new drugs for the oral treatment of leishmaniosis disease with fewer side effects and lower cost.
Read Full Article HTML DOI: 10.29328/journal.acst.1001032 Cite this Article Read Full Article PDF
Keywords:
Zanthoxylum chiloperone; Rutaceae; Paraguay; leishmaniosis; BALB/c
References
- Giménez A, Ruotti M, González N, Torales M, Rojas de Arias A. Epidemiological situation of leishmaniosis and perception of key actors in the department of Alto Paraná, Paraguay. Mem Inst Investig Health Science. 2017; 15(2):85-96.
- Pan American Health Organization. Leishmaniasis in the Americas: recommendations for treatment. Washington DC: PAHO, 2018. http://www.iris.paho.org/xmlui/handle/123456789/7704
- da Silva SS, Mizokami SS, Fanti JR, Costa IN, Bordignon J, Felipe I, Pavanelli WR, Verri WA Jr, Conchon Costa I. Glucantime reduces mechanical hyperalgesia in cutaneous leishmaniasis and complete Freund's adjuvant models of chronic inflammatory pain. J Pharm Pharmacol. 2018 Jun;70(6):768-777. doi: 10.1111/jphp.12896. Epub 2018 Mar 12. PMID: 29532470.
- Canese A, Canese A. Handbook of medical microbiology and parasitology. Assumption. 7th edition. 2012; 473.
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. Epub 2012 May 31. PMID: 22693548; PMCID: PMC3365071.
- Pan American Health Organization. Paraguayan. In: Health in the Americas. Editions OECD; 2012; 567–583.
- Chena L. Application of PCR for the detection of leishmania genus and complexes in different types of biological samples. Mem Inst Investig Health Science. 2013;9(1):45–51.
- University of Carabobo. Faculty of Health Sciences. D, Brito E, Simons MI. Salus: magazine of the Faculty of Health Sciences, University of Carabobo. Health. 2016; 20(2):24–9.
- Oddone R, Maciel Jd, Canese A, Velazquez GR, Meza T, Moran M. Clinical and serological follow-up of patients treated for cutaneous leishmaniosis. Mem Inst Investigate Cienc Health. 2007;3(1):39–44.
- Ministry of Public Health and Social Welfare. National Leishmaniasis Control Program. National Malaria Eradication Service (SENEPA). 2018. https://www.mspbs.gov.py/senepa/leishmaniosis.html.
- Portillo A, Vila R, Freixa B, Adzet T, Cañigueral S. Antifungal activity of Paraguayan plants used in traditional medicine. J Ethnopharmacol. 2001 Jun;76(1):93-8. doi: 10.1016/s0378-8741(01)00214-8. PMID: 11378288.
- Reithinger R, Dujardin DJC, Louzir H, Pirmez C, Alexander B, Brooker SJ. Cutaneous Leishmaniasis. Lancet Infect Dis. Sept 2007; 7(9):581-596.
- Osorio EJD, Montoya P, Arango GJ. Alkaloidal Natural Products with Antiprotozoal Activity. National university of Colombia. J Nat Prod. 2009; 72: 1355-1356.
- Ferreira ME, Cebrián-Torrejón G, Corrales AS, Vera de Bilbao N, Rolón M, Gomez CV, Leblanc K, Yaluf G, Schinini A, Torres S, Serna E, Rojas de Arias A, Poupon E, Fournet A. Zanthoxylum chiloperone leaves extract: first sustainable Chagas disease treatment. J Ethnopharmacol. 2011 Feb 16;133(3):986-93. doi: 10.1016/j.jep.2010.11.032. Epub 2010 Dec 4. PMID: 21134431.
- Ferreira ME, Nakayama H, de Arias AR, Schinini A, de Bilbao NV, Serna E, Lagoutte D, Soriano-Agatón F, Poupon E, Hocquemiller R, Fournet A. Effects of canthin-6-one alkaloids from Zanthoxylum chiloperone on Trypanosoma cruzi-infected mice. J Ethnopharmacol. 2007 Jan 19;109(2):258-63. doi: 10.1016/j.jep.2006.07.028. Epub 2006 Jul 29. PMID: 16949231.
- Thouvenel C, Gantier JC, Duret P, Fourneau C, Hocquemiller R, Ferreira ME, Rojas de Arias A, Fournet A. Antifungal compounds from Zanthoxylum chiloperone var. angustifolium. Phytother Res. 2003 Jun;17(6):678-80. doi: 10.1002/ptr.1137. PMID: 12820240.
- Ferreira ME, de Arias AR, Yaluff G, de Bilbao NV, Nakayama H, Torres S, Schinini A, Guy I, Heinzen H, Fournet A. Antileishmanial activity of furoquinolines and coumarins from Helietta apiculata. Phytomedicine. 2010 Apr;17(5):375-8. doi: 10.1016/j.phymed.2009.09.009. Epub 2009 Oct 29. PMID: 19879121.
- Nakayama H, Loiseau PM, Bories C, Torres de Ortiz S, Schinini A, Serna E, Rojas de Arias A, Fakhfakh MA, Franck X, Figadère B, Hocquemiller R, Fournet A. Efficacy of orally administered 2-substituted quinolines in experimental murine cutaneous and visceral leishmaniases. Antimicrob Agents Chemother. 2005 Dec;49(12):4950-6. doi: 10.1128/AAC.49.12.4950-4956.2005. PMID: 16304157; PMCID: PMC1315925.
- Serna ME, Maldonado M, Torres S, Schinini A, Lima AP, Pandolfi E, de Arias AR. Finding of leishmanicidal activity of 14-hydroxylunularin in mice experimentally infected with Leishmania infantum. Parasitol Int. 2015 Oct;64(5):295-8. doi: 10.1016/j.parint.2015.03.005. Epub 2015 Apr 3. PMID: 25843766.
- Cebriàn-Torrejòn G, Spelman K, Leblanc K, Muñoz-Durango K, Sandra Torijano S, Ferreira ME, Rojas de Arias A, Figadére B, Fournet A, Maciuk A, Grellier P, Cech NB, Poupon E. The antiplasmodium effects of a traditional South American remedy: Zanthoxylum chiloperone angustifolium against chloroquine resistant and chloroquine sensitive strains of Plasmodium falciparum. Rev Bras Farmacogn 2011; 21 (4):0102-695.
- Arias JL, Cebriàn-Torrejòn G, Poupon E, Fournet A, Couvreur P. Biodegradable polymeric Nano formulation based on the antiprotozoal canthin-6-one. J Nano part Res 2011; 13(12):6737–6746.
- Ruoti M, Oddone R, Lampert N, Orué E, Miles MA, Alexander N, Rehman AM, Njord R, Shu S, Brice S, Sinclair B, Krentel A. Mucocutaneous leishmaniasis: knowledge, attitudes, and practices among paraguayan communities, patients, and health professionals. J Trop Med. 2013;2013:538629. doi: 10.1155/2013/538629. Epub 2013 Apr 15. PMID: 23690792; PMCID: PMC3649269.
- Digital Biomedical Academy. Drugs used in the treatment of leishmaniasis. School of Medicine. Central University of Venezuela. Prognostic value of physiological changes associated with chemo-resistance in Leishmania. Portal Vitae. 2007; 33:1317-987.
- Salomón OD, Mastrángelo AV, Santini MS, Liotta DJ, Yadón ZE. La eco-epidemiología retrospectiva como herramienta aplicada a la vigilancia de la leishmaniasis en Misiones, Argentina, 1920-2014 [Retrospective eco-epidemiology as a tool for the surveillance of leishmaniasis in Misiones, Argentina, 1920-2014]. Rev Panam Salud Publica. 2016 Aug;40(1):29-39. Spanish. PMID: 27706386.
- Desjeux P. Leishmaniosis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004 Sep; 27(5):305–18.
- Yang X. Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J Agric Food Chem. 2008 Mar 12;56(5):1689-96. doi: 10.1021/jf0728101. Epub 2008 Feb 14. PMID: 18271544.
- Oddone R. Leishmaniosis visceral: a 101 años del primer caso diagnosticado en las Américas. Mem Inst Investig Cienc Salud. 2012; 10(1): 100-104.
- Piccolo L, Pérez E, Álvarez L, Wang C, Sancho M. Leishmaniasis: Therapeutic options in the pediatric population. Med Legal Costa Rica. 2018; 35(1):2215-5287.
- Degen R, Soria N, Ortiz M. Problems of common names of commercialized medicinal plants in Paraguay. Fac Cien Quim Univ Nac Asu. 2005; 21(1):11–6.
- Saz-Peiró P, Ortiz-Lucas M. Naturopathic medicine, a vision of scientific research in recent years. Med Nat. 2007; 1(2):11-8.
- Hernández A. Phytotherapy. Scientific and legal bases for its application. BLACPMA. 2005; 4(4):71-4.
- Haumeil JCC, Renaud PA. The evaluation of new molecular entities. Biol Pharm Bull. 2002; 25(12):1600–3.
- Basualdo I, Soria N. Paraguayan Herbal Pharmacopoeia: Species of folk medicine used to combat diseases of the respiratory system. Fac Cienc Quim UNA. 2017; 3(2): 197–238.
- Cordero J, Boshier D. Trees of Central America: a manual for extension workers. Tropical Agricultural Research and Teaching Center. Bib Orton IICA / CATIE 2003; 1079.
- Council for International Organizations of Medical Sciences. CIOMS/ICLAS. International Guiding Principles for Biomedical Research Involving Animals. 2012.
- Artzi O, Sprecher E, Koren A, Mehrabi JN, Katz O, Hilerowich Y. Fractional Ablative CO2 Laser Followed by Topical Application of Sodium Stibogluconate for Treatment of Active Cutaneous Leishmaniasis: A Randomized Controlled Trial. Acta Derm Venereol. 2019 Jan 1;99(1):53-57. doi: 10.2340/00015555-3058. PMID: 30281141.
- Parvizi MM, Handjani F, Moein M, Hatam G, Nimrouzi M, Hassanzadeh J, Hamidizadeh N, Khorrami HR, Zarshenas MM. Efficacy of cryotherapy plus topical Juniperus excelsa M. Bieb cream versus cryotherapy plus placebo in the treatment of Old World cutaneous leishmaniasis: A triple-blind randomized controlled clinical trial. PLoS Negl Trop Dis. 2017 Oct 5;11(10):e0005957. doi: 10.1371/journal.pntd.0005957. PMID: 28981503; PMCID: PMC5655399.
- Jaffary F, Nilforoushzadeh MA, Siadat A, Haftbaradaran E, Ansari N, Ahmadi E. A Comparison between the Effects of Glucantime, Topical Trichloroacetic Acid 50% plus Glucantime, and Fractional Carbon Dioxide Laser plus Glucantime on Cutaneous Leishmaniasis Lesions. Dermatol Res Pract. 2016;2016:6462804. doi: 10.1155/2016/6462804. Epub 2016 Apr 11. PMID: 27148363; PMCID: PMC4842369.
Figures:
Similar Articles
-
The combination of very-small size proteoliposomes and alum is a safe adjuvant alternative for inducing anti-EGF antibodies: a preclinical studyMabel Cruz Rodríguez,Gretchen Bergado Báez,Yerandy Hechevarría Luna,Diana Rosa Hernández Fernández,Addys González Palomo,Narjara González Suárez,Carlos Yordan González Castillo,María del Carmen Luzardo Lorenzo,Lisset Chao García,Belinda Sánchez Ramírez*. The combination of very-small size proteoliposomes and alum is a safe adjuvant alternative for inducing anti-EGF antibodies: a preclinical study. . 2022 doi: 10.29328/journal.acst.1001029; 6: 018-030
-
Determination of the in vivo activity of leaves extract of Zanthoxylum Chiloperone var. Angustifolium (Tembetary hú) orally and intralesionally administered to BALB/c mice experimentally infected with LeishmaniaElva Serna*,Marisel Maldonado,Nilsa González. Determination of the in vivo activity of leaves extract of Zanthoxylum Chiloperone var. Angustifolium (Tembetary hú) orally and intralesionally administered to BALB/c mice experimentally infected with Leishmania. . 2022 doi: 10.29328/journal.acst.1001032; 6: 038-043
Recently Viewed
-
Unveiling Disparities in WHO Grade II Glioma Care among Physicians in Middle East and North African (MENA) Countries: A Multidisciplinary SurveyFatimah M Kaabi,Layth Mula-Hussain*,Shakir Al-Shakir,Sultan Alsaiari,Leonidas Chelis,Renda AlHabib,Sara Owaidah,Renad Subaie,Marwah M Abdulkader,Ibrahim Alotain. Unveiling Disparities in WHO Grade II Glioma Care among Physicians in Middle East and North African (MENA) Countries: A Multidisciplinary Survey. Arch Cancer Sci Ther. 2026: doi: 10.29328/journal.acst.1001048; 10: 001-005
-
Screening for Depressive Symptoms in Clinical and Nonclinical Youth: The Psychometric Properties of the Dutch Children’s Depression Inventory-2 (CDI-2)Denise HM Bodden*,Yvonne Stikkelbroek,Daan Creemers,Sanne PA Rasing,Elien De Caluwe,Caroline Braet. Screening for Depressive Symptoms in Clinical and Nonclinical Youth: The Psychometric Properties of the Dutch Children’s Depression Inventory-2 (CDI-2). Insights Depress Anxiety. 2025: doi: 10.29328/journal.ida.1001047; 9: 028-039
-
Penile Fracture: The “Cracking” Sound and Intra-operative Tunica Albuginea RepairAyoub Mamad*,Mohammed Amine Bibat,Mohammed Amine Elafari,Youssef Maachi,Amine Slaoui,Tarik Karmouni,Abdelatif Koutani,Khalid Elkhader. Penile Fracture: The “Cracking” Sound and Intra-operative Tunica Albuginea Repair. J Clin Med Exp Images. 2026: doi: 10.29328/journal.jcmei.1001038; 10: 001-002
-
Transumbilical Single-incision Hiatal Hernia Repair and Nissen Fundoplication in situs Inversus Totalis: A Rare Case ReportQing Cao,Chen Kang,Kang Gu,Yin Peng,Yang Lv,Xu-Zhong Ding,Peng Li*. Transumbilical Single-incision Hiatal Hernia Repair and Nissen Fundoplication in situs Inversus Totalis: A Rare Case Report. Adv Treat ENT Disord. 2026: doi: 10.29328/journal.ated.1001017; 10: 001-003
-
NAD⁺ Biology in Ageing and Chronic Disease: Mechanisms and Evidence across Skin, Fertility, Osteoarthritis, Hearing and Vision Loss, Gut Health, Cardiovascular–Hepatic Metabolism, Neurological Disorders, and MuscleRizwan Uppal,Umar Saeed*,Muhammad Rehan Uppal. NAD⁺ Biology in Ageing and Chronic Disease: Mechanisms and Evidence across Skin, Fertility, Osteoarthritis, Hearing and Vision Loss, Gut Health, Cardiovascular–Hepatic Metabolism, Neurological Disorders, and Muscle. Ann Clin Endocrinol Metabol. 2026: doi: 10.29328/journal.acem.1001032; 10: 001-009
Most Viewed
-
Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization)Dasse Sery Romuald*, KL Siransy, N Koffi, RO Yeboah, EK Nguessan, HA Adou, VP Goran-Kouacou, AU Assi, JY Seri, S Moussa, D Oura, CL Memel, H Koya, E Atoukoula. Impact of Latex Sensitization on Asthma and Rhinitis Progression: A Study at Abidjan-Cocody University Hospital - Côte d’Ivoire (Progression of Asthma and Rhinitis related to Latex Sensitization). Arch Asthma Allergy Immunol. 2024 doi: 10.29328/journal.aaai.1001035; 8: 007-012
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037
-
Snow white: an allergic girl?Oreste Vittore Brenna*. Snow white: an allergic girl?. Arch Asthma Allergy Immunol. 2022 doi: 10.29328/journal.aaai.1001029; 6: 001-002
-
Cytokine intoxication as a model of cell apoptosis and predict of schizophrenia - like affective disordersElena Viktorovna Drozdova*. Cytokine intoxication as a model of cell apoptosis and predict of schizophrenia - like affective disorders. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001028; 5: 038-040

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."