More Information
Submitted: 16 September 2019 | Approved: 26 September 2019 | Published: 27 September 2019
How to cite this article: Emeraude NC, Saint-Cyr Sylvestre PD, Clotaire ND, Sarah B, Ismael K, et al. Results of chemotherapy in the treatment of chronic lymphoid leukemia in Black Africa: Experience of Côte d’Ivoire. Arch Cancer Sci Ther. 2019; 3: 045-048.
DOI: 10.29328/journal.acst.1001008
Copyright License: © 2019 Emeraude NC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Chronic lymphoid leukemia; Polychemotherapy; Black Africa
Results of chemotherapy in the treatment of chronic lymphoid leukemia in Black Africa: Experience of Côte d’Ivoire
N’dhatz Comoe Emeraude1, Packo Dieu-le-veut Saint-Cyr Sylvestre1*, Nanho Danho Clotaire1, Bognini Sarah2, Kamara Ismael1, Boidy Kouakou1 and Koffi Kouassi Gustave1
1Department of Clinical Hematology, University of “Felix Houphouet Boigny”, Abidjan, Côte d’Ivoire
2Department of Clinical Biology, University of “Felix Houphouet Boigny”, Abidjan, Côte d’Ivoire
*Address for Correspondence: Dr. Packo Dieu-le-Veut Saint-Cyr Sylvestre, Department of Clinical Hematology, University of “Felix Houphouet Boigny”, Abidjan (Côte d’Ivoire), P.O. Box 1439, Abidjan 04, Cote d’Ivoire, Tel: 0022589542209; Email: [email protected]
Background: The treatment of chronic lymphoid leukemia currently uses news drugs which are more expensive in our countries. Its why, the results of chemotherapy remains a challenge in our sector.
Aims: To evaluate the place of polychemotherapy in the treatment of chronic lymphoid leukemia in black Africa.
Methods: It was a prospective, descriptive, analytic and non-comparative study, concerning the records of patients with chronic lymphoid leukemia treated and followed at the department of clinical hematology in Abidjan.
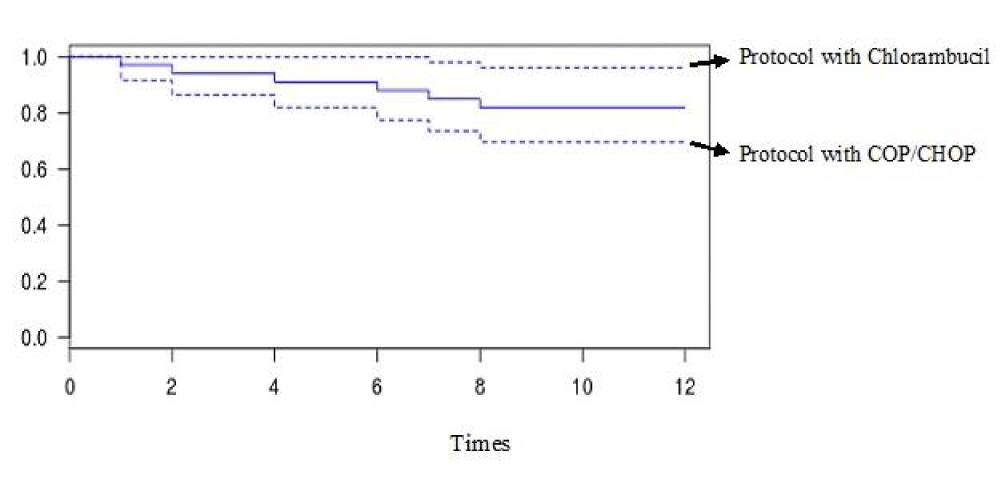
Results: We included 56 patients. The average age was 62 years with extremes of 38 and 84 years. The sex ratio was 0.8 in favor of female. The clinical signs noted a tumor syndrome among which splenomegaly, classified stage III (46, 43%) and adenopathy (64, 29%). Biologically, we observed a blood lymphocytosis (50%), an anemia (39.29%) and a thrombocytopenia (62.50%). The majority of patients were classified stage A of BINET (51.79%). The COP protocol (44.64%) and the monochemotherapy with chlorambucil (39.29%) were the most used. The therapeutic response of polychemotherapy was low (12.5%) compared to 35, 71% for monochemotherapy (p = 0.0001) with overall survival significantly better in monochemotherapy. The outcome of patients used polychemotherapy were more adverse that of patients used chlorambucil alone (p = 0,003). The overall probability of survival at 12 months was 90, 9% for patients who used monochemotherapy and 63, 4% for polychemotherapy.
Conclusion: Polychemotherapy in chronic lymphoid leukemia of black African has an adverse therapeutic response hence the interest of using new therapeutic possibilities.
Chronic lymphoid leukemia (CLL) is the most common leukemia in western countries [1]. The median of patients during the diagnosis is 64 years-old and 10% of patients are diagnosed before 50 years old [1,2]. The prognosis of this disease is heterogeneous, since some patients die two years after diagnosis while others survive for more than 20 years and die of non-disease related causes [3]. Regarding treatment, according to the recommendations of the national cancer institute (NCI), polychemotherapy remains broadened to a group of patients in stages B or C of BINET and refractory forms of initial forms A of BINET [4]. In addition, the therapeutic progress observed during the CLL has been marked by the discovery of news therapeutics such as fludarabine, monoclonal antibodies and purine derivatives. However, since the opening of department of hematology in Côte d’Ivoire, the choice of the use of monochemotherapy and polychemotherapy remain diversified with sometimes controversial results [5]. The objective of this study was to evaluate the place of polychemotherapy in the treatment of CLL of black African subjects and to identify their therapeutic and evolutionary characteristics.
It was a retrospective, descriptive, analytic and non-comparative study with duration of two years (From August 2017 to September 2019), conducted at the department of clinical hematology in Abidjan. This study concerned the files of black African patients with CCL, treated and followed during this period. 56 patients were included in our study. The recruitment was systematic random. The data were collected using a survey sheet prepared and standardized. The parameters studied were epidemiological, clinical, biological and therapeutic. The therapeutical’s parameters had concerned the patients who were treated using CHOP, COP or Chlorambucil protocols according to the posology and the following modes of administration:
CHOP protocol: Cyclophosphamide: 750 mg/m2, IV day 1
H = Doxorubicine: 50 mg/m2, IV day1
Oncovin® ou Vincritine: 1,4 mg/m2, IV day 1
Prednisone: 40 mg/m2 Per os day 1 to day 5
Day 1 = Day 21
COP protocol: Cyclophosphamide: 300 mg/m2, IV day 1
Oncovin® ou Vincritine: 1,4 mg/m2, day 1
Prednisone: 60 mg/m2, day 1 to day 5
Day 1 = Day 21
Chlorambucil Protocol: Chlorambucil 0,1 mg/kg en PO continuous.
The total number of cycles of treatment was 6 or 8 cycles for COP or CHOP protocol and 12 cycles for chlorambucil protocol. As for the therapeutic responses, we defined the Complete Response as the disappearance of clinical signs and a number greater than 4G/L of blood lymphocytes. Partial Response was defined as a decrease of 50% of tumor and of lymphocytosis. The progressing disease involved increased tumor syndrome and lymphocytosis by more than 50%. This study was conducted in compliance with all the applicable institutional ethical guidelines for the care, welfare and use of animals.
Data entry and statistical analyses
Data analysis was performed using EPI-INFO 6.04b software at the significance level of 5%. The independence and percentage comparison tests were performed using the chi-square test (X²). The calculation of survival was done according to the Kaplan-Meir method, taking into account the different prognostic factors. The comparison of the survival curves was made using “log-rank” test.
Our sample was constituted of 56 patients. The tables 1 and 2 summarize respectively the descriptive characteristic and Therapeutical protocol, Therapeutical responses and Outcome of patients. As for the table 3, it shows the analytical characteristic of therapeutical responses of patients. The figure 1 gives the overall survival curve of patient.
| Table 1: Descriptive characteristic. | |
| Variables | Numbers (%) |
| Epidemiological Data | |
| Ages (years): average and extremes 62 [38 and 84] | |
| 38-59 | 32 (57,14) |
| 60-84 | 24 (42,85) |
| Sex | |
| Female | 31 (55,36) |
| Male | 25 (44,64) |
| Clinical manifestations | |
| Performance status | |
| 0-2 | 48 (14,28) |
| 3-4 | 8 (85,72) |
| > Tumor syndrome | |
| Splenomegaly (stage of Hacket) | |
| 1-2 3 4-5 |
13 (23,22) 26 (46,43) 17 (30,35) |
| lymphadenopathy | 36 (64,29) |
| Biological manifestations | |
| Blood lymphocytosis | |
| < 75 75-80 > 80 |
10 (17,86) 18 (32,14) 28 (50) |
| Hemoglobin level (g/dl) | |
| <10 >10 |
22 (39,29) 34 (60,71) |
| Blood platelets level (G/L) | |
| <150 ≥150 |
35 (62,50) 21 (37,50) |
| Binet stadification | |
| A B and C |
29 (51,79) 27 (48,21) |
| Table 2: Therapeutical protocol, Therapeutical responses and Outcome of patients | |
| Variables | Numbers (%) |
| Therapeutical protocol | |
| CHOP | 9 (16,07) |
| COP | 25 (44,64) |
| Chlorambucil | 22 (39,29) |
| Therapeutical responses | |
| Complete response | 27 (48,21) |
| Partial response | 20 (35,71) |
| Progressive disease | 7 (12,5) |
| Out come | |
| Living and on treatment | 15 (26,78) |
| Lost to followup | 22 (39,28) |
| Dead | 19 (39,92) |
| Table 3: Analytical characteristic of therapeutical responses of patients. | |||
| CHOP/COP | Chlorambucil | p | |
| Numbers (%) | Numbers (%) | ||
| RP | 20 (35,71) | 3 (5,35) | 0,001 |
| CR | 7 (12,5) | 15(35, 71) | |
| PD | 7 (12,5) | 4 (7,14) | |
| Living and on treatment | 17(50) | 20(90,90) | 0,003 |
| Death | 17(50) | 2(9,10) | |
Figure 1: Overral survival curve.
Our study was prospective, descriptive, analytic and non-comparative. It concerned the black Africans patients with CCL during the period from August 2017 to September 2019. It consisted to evaluate the place of polychemotherapy in the treatment of CCL. This present study showed that CLL is pathology of the elderly subject similar to the literature data [1]. We noted a predominance of female with ratio sex of 0,8. Halker, et al. and Bastin, et al. had observed respectively a sex ratio 2 and 1,4 in favor of male [6,7]. These differences with our study could be explained by the size of the sample. Clinically, blood lymphocytosis (23, 21%) and splenomegaly (39, 29%) were the most frequent and our data were similar to those of Merle, et al. [8]. The African literature showed that, CCL of African black is very tumoral and this seems to be related to a consultation delay [9,10]. The disease has a slow and chronic course; patients can live for ten years or can have a relatively normal life. Indeed in our study, the majority of our patients had a good general condition (85, 7%). Our results were identical of Koffi, et al. [9]. Lymphadenopathy were present clinically in 35.71% of cases, and defined the features of CCL of African black which splenomegaly is more predominate than lymphadenopathy. These data were found by all the previous studies [8-10]. Biologically, blood lymphocytosis was variable from one patient to another with a lymphocyte count of up to 200 G/L according to Merle, et al. [8]. Anemia is usually related to bone marrow failure. But it can be due to autoimmune hemolytic or an erythroblastopenia The majority of our patients were stage A of BINET (51.79%), similar to the study of Koffi, et al. and Ayemou, et al. [5,9]. Stage A of BINET is attributed to the independent factor of mortality and associated with a good factor. Indeed, the prognosis of the disease is a factor impacting the clinical, biological characteristics, the treatment, the therapeutic responses and the survival of the patients in our environment in Africa. Polychemotherapy was performed in 34 patients and 22 patients were treated by mono chemotherapy including chlorambucil. The choice of our treatment related to the stage of BINET, the presence of comorbidity and the financial means of patients. Apart from these conventional therapeutic protocols, the treatment of CLL has been disrupted since the discovery of new molecules such as monoclonal antibodies and fludarabine. These news drugs are used in particular during the advanced stages B and C and the emergence of refractory forms. Indeed, these news therapeutic molecules are available in Africa in our hospitals but inaccessible for the majority of our patients because of high cost. Thus conventional chemotherapy remains the only therapeutic alternative for the management of our patients. Therapeutically, 51.79% of our patients were CR while 48.21% had PR. We noted 33.92% of deaths, 39.29% of cases alive and 26.79% of Lost to follow-up. The CCL remains primarily an incurable disease, which can be cured at the cost of an allogeneic hematopoetic sterm cell graft. But, the age restricts this indication. Nowadays, the evaluation of the therapeutic response uses classical criteria of Complete Response (CR), Partial Response and Progressive Disease, but also phenotypic and medullar remission. The quality of the CR is evaluated by PCR on the Ig heavy chain genes and seems to be an essential factor of the prognosis after therapeutic intensification. Such explorations are inaccessible in our exercise condition, so that CR remains purely clinical and long-term, anecdotal RC. The analytical characteristic showed in our study that therapeutic response rates with polychemotherapy was worse comparated to the results of monochemotherapy. Our results were similar to the study of Lepetre, et al. [10]. Another meta-analysis study involving 2,000 patients from 10 trials confirms the efficacy of chlorambucil compared to combinations with ± doxorubicin alkylating agents [11,12]. However, the study of Leblond, et al. had showed that monochemotherapy by chlorambucil give worse results with less 10% of CR [13]. These differences could be explained by the indication of use of chemotherapy during CCL. Endeed, the polychemotherapy protocol in CLL applies to advanced stage of BINET B and C where complications are already established, unlike the monochemotherapy which is used at an early stage of the disease with a patient in good general state. In the CCL-80 and CCL-85 protocols, using polychemotherapy protocols, 60% response rates in advanced stages B were higher than that in our study [14]. However, in no study, this difference in efficacy has resulted in an increase in survival. The efficacy in terms of survival of the monochemotherapy on multidrug therapy observed during our study was confirmed by Jaksi, et al. using high doses of chlorambucil (10 mg/m²/day) until the RC [15].
Polychemotherapy in chronic lymphoid leukemia of African black has an adverse therapeutic response hence the interest of using new therapeutic possibilities.
The authors welcome the collaboration the team of department of clinical hematology of Yopougon Teaching Hospital for their contribution during the management of these patients.
Funding
This study was not funded and had no personal or financial relationship that may have improperly influenced the writing. It was done as part of the professional activities of the authors. These authors are supervised by the Department of Clinical Hematology of Yopougon Teaching Hospital.
Authors contributions
The idea of this study was provided from Koffi Kouassi Gustave. He designed Drs N’dhatz Comoe Emeraude and Packo Dieu-le-veut Saint-cyr Sylvestre as a main investigator and proceeded to the selection of case, the interview, the documentary research and the writing of the manuscript. Professor Nanho Danho Clotaire, Drs. Kamara Ismael, Bognini Sarah and Boidy Kouakou revised the manuscript and provided additional information to enrich it. Koffi Kouassi supervised the study and did the final correction. All authors have read and approved the final version of the manuscript.
- Evrard S, Gaussem P, Helley D, Darnige L. Facteurs pronostiques de la leucémie lymphoide chronique: Apports des marqueurs biologiques récents. Ann Biol Clin. 2015; 63: 589-597.
- Société française d'Hématologie. Leucémie Lymphoïde chronique. Hématologie. 2007; 13: 348-351.
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl Journ Med. 2005; 352: 804-815.
- Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981; 48: 198-206. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7237385
- Koffi KG, Nanho DC, Tolo A, N'Dathz E, Kouakou B, et al. La leucémie lymphoïde chronique du Noir en Afrique subsaharienne: caractéristiques cliniques thérapeutiques et pronostiques (cas de la Côte d'Ivoire). Bull Cancer. 2009; 96: 901-916.
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, el al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on chronic lymphocytic leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008; 111: 5446-5456. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18216293
- Bastin Ch. Les leucémies lymphoïdes chroniques; la diversité des cas et leur évolution. Cahiers de l'analyse des données. 1976; 419-440.
- Merle B. leucémie lymphoïde chronique: diagnostic, évolution, pronostic, traitement. Rev Prat. 1993; 43: 2713-2717.
- Toutoukpo Y, Tea N, Drain J. La leucemie lymphoïde chronique en Cote d'Ivoire (a propos de 69 cas). Med Trop. 1981; 51: 417-420.
- Lepretre S, Van Den Neste E. Place de la chimiothérapie dans la leucémie lymphoïde chronique. Hématol. 2006; 12: 28-34.
- Jakši B, Pejša V, Ostoji-Koloni S, Kardum-Skelin I, Baši Kinda S, Coha B, et al. Guidelines for Diagnosis and Treatment of Chronic Lymphocytic Leukemia. Krohem B-Cll 2017. Acta Clin Croat. 2018; 57: 190-215. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30256032
- CLL Trialists' Collaborative Group. Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. J Nat Cancer Inst. 1999; 91: 861-868. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10340906
- Leblond V, Delmer A. Prise en charge de la leucémie lymphoïde chronique en première ligne. Correspondances en onco-hématologie. 2009; 4: 122-126.
- Dighiero G, Maloum K, Desablens B, Navarro M, Leblay R, et al. Chlorambucil in indolent chronic lymphocytic leukemia. French Cooperative Group on Chronic Lymphocytic Leukemia. N Engl J Med. 1998; 338: 1506-1514. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9593789
- Jaksi B, Brugiatelli M. High dose continuous chlorambucil vs intermittent chlorambucil plus prednisone for treatment of B-CLL--IGCI CLL-01 trial. Nouv Rev Fr Hematol. 1988; 30: 437-442. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3065740