More Information
Submitted: 11 April 2020 | Approved: 28 April 2020 | Published: 29 April 2020
How to cite this article: Panico VJA, Simardi LH, Faria EF, Sotelo R, Suarez R, et al. Electrocoagulation with greased lidocaine gel 2% as hemostatic maneuver after minimally invasive partial nephrectomy: Experimental and preliminary clinical results. Arch Cancer Sci Ther. 2020; 4: 019-023.
DOI: 10.29328/journal.acst.1001017
Copyright License: © 2020 Panico VJA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Electrocoagulation with greased lidocaine gel 2% as hemostatic maneuver after minimally invasive partial nephrectomy: Experimental and preliminary clinical results
Vinicius JA Panico1,2, Lucila H Simardi3, Eliney F Faria4,5, Rene Sotelo6, Ruben Suarez7, Diego Abreu7, Andre Meirelles8, Edison Schneider8, Hamilton C Zampolli1 and Marcos Tobias-Machado1*
1Instituto do Cancer dr Arnaldo Vieira de Carvalho, São Paulo, Brazil
2Hospital do Cancer de Londrina, Londrina, Parana, Brazil
3Faculadade de Medicina do ABC, Brazil
4Hospital Felicio Rocho, Belo Horizonte, MG, Brazil
55Hospital do Amor, Fundação Pio XI, Barretos, São Paulo, Brazil
6University of South California, LA, California, USA
7Hospital Pasteur, Montevideo, Uruguay
8Pontificie Universidade Catolica de Campinas, Sao Paulo, Brazil
*Address for Correspondence: Marcos Tobias-Machado, Rua Continental 50, Sao Bernardo do Campo, Sao Paulo, ZIP 09750-060, Brazil, Email: [email protected]
Methods: Experimental phase: Performed a partial nephrectomy off clamp in pig model followed by cauterization of lidocaine gel 2% with different power (control, 30W, 50W and 100W) in the kidney resection bed to evaluate efficacy and deep injury extension.
Clinical phase: 20 patients submitted to laparoscopic or partial nephrectomy for low risk RENAL score were utilized greased lidocaine gel 2% with 50W in cautery scalpel to hemostasis of renal parenchima to validate efficacy and safety.
Results: Experimental study shows that this technique is effective and promote better hemostasis with 50W and 100W, with deep injury of less than 3 mm.
Clinical study confirm efficacy, good control of hemorrage, few complications and no transfusion. Minimal changes in hematocrit, haemoglobin and creatinine were observed.
Conclusion: In this preliminary experience the use of this new alternative to hemostasis for low risk partial nephrectomy was satisfactory and with good intra and postoperative results.
The best advantages were safety in terms of the depth thermal injury, low cost and absence of artifacts over the resection area observed at CT scan postoperatively.
The standard treatment for patients with localized renal mass is surgical excision. In the last 2 decades, nephron sparing surgery emerge as equivalent alternative to radical nephrectomy in most cases of renal cell carcinoma [1]. More recently, technologies such as thermal ablation, laparoscopy or robotic assisted approach extended the therapeutic arsenal [2].
In the absence of level I evidence, treatment settings are subject to changes that may be proportional to the standard of training, comfort and level of individual experience [3].
RENAL scoreis an interesting instrument to classify kidney tumors into 3 groups of complexity (low, intermediate and high) using the CT scan to reproduce the tumor anatomy. The criteria utilized were size, exophytic/endophytic, nearness to collecting system sinus (mm), anterior/posterior, location relative to polar lines and if the mass is hilar. Some research show that these scoreallowi to predictintra and postoperative risks of surgical complications [4,5].
Among surgical complications of partial nephrectomy, hemorrhage is the most common. Renal hemorrhage after accidental trauma or during operative procedures can be severe and difficult to control [6]. The surgeon is responsible for adequate control of the renal hilum during resections of lesions and or bleeding control of traumatic renal parenchyma injury, to prevent complications and even death [7]. Clamping the vascular pedicle is a standarttechnique used to prevent massive hemorrhage. If patient have a good basal renal function the ideal time of warm ischaemia is not exceeding 30 minutes. We know that clamping time longer than recommended can be harmful to the remaining renal parenchyma [8]. The volume of blood lost and warm ischemia time are decisive in the postoperative prognosis of renal function [9].
During conventional renal resection, despite the control of the renal hilum for partial resection, bleeding still occurs. Several surgeons use total or selective vascular clamping maneuvers in attempt to decrease blood loss [10]. However, this manouver is accompanied by temporary organ ischemia, which can be deleterious when longer than 30 minutes [8].
Although the morbidity and mortality of these procedures has decreased, Advancement of imaging methods promote diagnosis of smaller asymptomatic lesions suitable for nephron-preserving surgery like enucleation or partial resection [11,12]. In some cases with minor renal parenchyma injury, we can opt for resection without pedicle clamping, application of hemostatic agents and sometimes no suture of kidney parenchima.
Despite advances in surgical techniques for hemostasis after partial nephrectomy, one limiting factor is the cost of synthetic hemostatics, which limits us to their use in the public hospitals and some times in the private one.
The aim of this report were to offer experimental and preliminary clinical data about a new hemostatic low cost technique for hemostasis after minimally invasive partial nephrectomy.
This paper is composed by 2 phases. Initially we test the potency of cautery and extension of the tissue lesion in pigs. After technical standardization suggested by experimental study and evaluation of amount of smoke to have good visualization we initiate our experience in humans.
Experimental phase
This pilot study was performed in a laparoscopy training laboratory, previously approved by the animal ethics committee of the minimally invasive surgery laboratory at IRCAD Latin America –Barretos-Brazil.
We performed a partial nephrectomy off clamp in pig models followed by cauterization of lidocaine gel 2% in the bleeding resection bed to evaluate the safety and efficacy of the procedure in renal parenchyma.
Control: Cauterization of the renal bleeding of the renal parenchyma without use lidocaine gel 2%
Lesion: Circular wedges
Radius: 1.0 a 1.8 cm
Deep: 0.3 a 0.9 cm
Power:
30W of potency with Spray (no lesion)
30W of potency with Spray and with Lesion
50W of potency with Spray and with Lesion
100W of potency with Spray and with Lesion
Intervention: Cauterization of the renal bleeding of the renal parenchyma with use lidocaine gel 2%
Lesion: Circular wedges
Radius: 1.0 a 1.8 cm
Deep: 0.3 a 0.9 cm
Power:
30W of potency with Spray (no lesion)
30W of potency with Spray and with Lesion
50W of potency with Spray and with Lesion
100W of potency with Spray and with Lesion
Clinical phase
After assigned inform consent for procedure we select patients with small renal mass and RENAL score of low complexity candidates to perform off clamp laparoscopic or robotic partial nephrectomy between January 2018 to January 2020. Patients with severe comorbidity (ASA II ou IV) were excluded. Antiaggregant drugs were suspended 10 days before surgical procedure.
After tumor resection it was injected lidocaine gel 2% through a plastic catheter over the resected surface. Cautery was calibrated to 50W (chose after 4 first cases because produce less amount of surgical smoke than 100W) and triggered to burn gel until a firm clust emerged to complete hemostasis of kidney surface. After that no hemostatic parenchymal suture was planned except if hemostasis could not achieved. If possible Gerota’s fascia was sutured to cover the defect and reconstitution of anatomy.
Patients had sample of blood to measure hematocrit/haemoglobin and creatinine 12 hours after surgery to evaluate blood loss and renal function.
All patients receive low molecular weight heparin for thromboembolism prevention for 4 weeks starting 12h after procedure if no significant bleeding occurred.
Experimental results
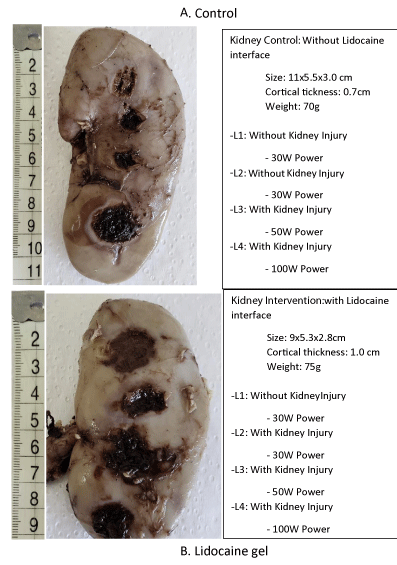
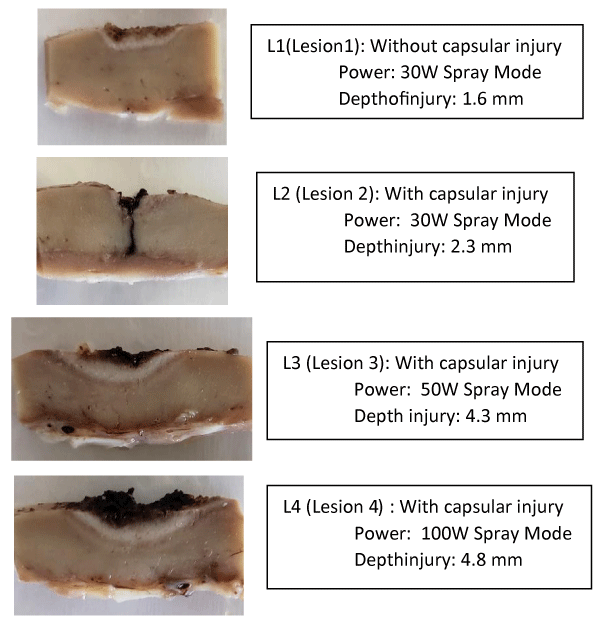
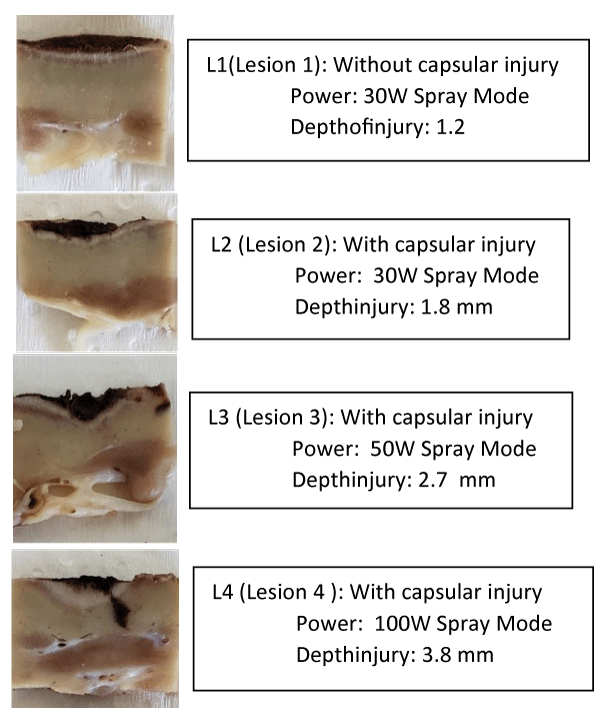
Macroscopically, it is clear that the cauterization of the bloody bed of the lesion with a lidocaine gel 2% interface injured less the healthy parenchyma in depth when compared to the same potency without the use of lidocaine (Figures 1-3 and Tables 1,2).
Figure 1: Macroscopic view of control kidney submitted to different power of eletrocautery. A. control of burned kidney injury without lidocaine gel; B. lidney injury burned with lidocaine gel.
Figure 2: Cortical macroscopic view: Kidney control (without lidocaine interface).
Figure 3: Cortical macroscopic view: Kidney Intervention (with lidocaine interface).
| Table 1: Lesion dimensions, power of electro cauterization in “spray” mode and depth of the injury of healthy parenchyma for successful hemostasis, without using lidocaine gel 2% as the conduction interface. | ||||
| Kidney Control | Size | Depth | Power (Spray Mode) | Depth injury |
| Lesion 1 | 1.2x1.0 cm | - | 30W | 0.16 cm |
| Lesion 2 | 1.0x1.0 cm | 0.4 cm | 30W | 0.23 cm |
| Lesion 3 | 1.0x1.0 cm | 0.4 cm | 50W | 0.43 cm |
| Lesion 4 | 1.5x1.8 cm | 0.5 cm | 100W | 0.48 cm |
| Table 2: Lesion dimensions, power of electro cauterization in “spray” mode and depth of the injury of healthy parenchyma for successful hemostasis, with using lidocaine gel 2% as the conduction interface. | ||||
| Kidney Intervention | Size | Depth | Power | Depth Injury |
| Lesion 1 | 1.6x2.0 cm | - | 30W | 0.12 cm |
| Lesion 2 | 0.9x1.5 cm | 0.3 cm | 30W | 0.18 cm |
| Lesion 3 | 1.6x2.5 cm | 0.4 cm | 50W | 0.26 cm |
| Lesion4 | 1.5x1.5 cm | 0.4 cm | 100W | 0.38 cm |
The time to obtain hemostasis without lidocaine was longer, as it does not suffer the physical tamponage that occurs when the lidocaine interface is dehidratate when submitted to different power of energy, lidocayne undergoes dehydration, making a more solid crust buffering the bleeding surface. Lidocaine is water-soluble and has electrical conductivity characteristics, in addition to acting as a sealant when the water evaporates, it is believed to act as a protector of energy dissipation for the healthy parenchyma, with a smaller halo of energy dissipation being seen in microscopy using interface of lidocaine.
According to this results we observe that potencies of 50W and 100W over lidocaine gel promote faster coagulation, minimal tissue injury with acceptable deep penetration.
Clinical results
Demographic data
It was included 20 cases of partial nephrectomy in multiple American centers.
Mean age was 55 years (40-65), 12 male and 8 female. Regarding comorbidities 8(40%) had controlled hypertension and 5(25%) had type II diabetes controlled with oral medication.
Thirteen cases underwent pure laparoscopic and 7 underwent robotic transperitoneal technique. In seventeen cases access was transperitoneal and in 3 cases retroperitoneal.
Report of clinical and laboratorial results
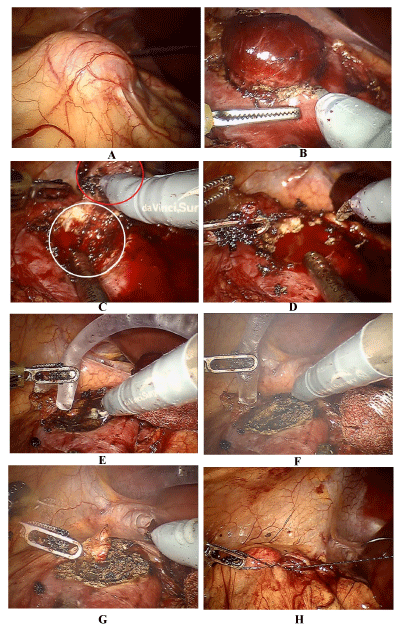
Hemostasis with greased lidocaine with no suture were obtained in all cases (Figure 4) Mean blood loss was 350 (150-500) ml. No intraoperative complications occurred. The average drain output in the first 24 hours was less than 100ml of serohematicoid liquid in all patients. No reoperation or readmition due to bleeding or other postoperative complications were observed. 15(75%) cases discharged after 24 h and other 5(15%) cases were discharged until 72 hours after surgery.
Figure 4: Endoscopic view of 3 cm exofitic kidney tumor; B,C,D. robotic minimal margins off clamp robotic partial nephrectomy; E. application of lidocaine gel 2% throught plastic catheter over kidney defect in the resection area; F,G. burning lidocaine and formation of crust that cover defect; H: suture of Gerota fascia to cover the defect.
Mean variation in hematocrit was 2(0-6), mean variation of haemoglobin as 1ng/ml (0.5-3.0) and mean variation of creatinine was 0.3ng/ml (0-0.5).
All cases reveled renal cell carcinoma without compromised margins. Postoperative CT scan did not present any artifact mimicking tumor or sign of residual cancer.
Electrocoagulation using lidocaine gel 2% in the bed bleeding after liver resection was first described by Mattos Filho, et al. in 2009 in rabbits [13] and reproduced by Petroianu in 2011 in humans [14]. Based in this principals we hypothetize that this kind of hemostasis will be suitable after enucleation of small renal tumors.
Cautherization after partial nephrectomy can achieve good hemostasis for vessels with thin caliber. Then, we select only small renal masses, exophytic and distant from collecting system sinus (classified as Low Complexity by RENAL Score) for these preliminary experience.
To the best of our knowledge this is the first description of this technique applied to renal surgery.
The technique was developed on swine model at different levels of power in the electrocautery in order to determine the depth of thermal damage by the process. The preliminary results provide us with security for the use of this as there no unwanted transmission of energy to deep structures on the face of tumor enucleation.
In humans electrocoagulation of 2% lidocaine gel in the bleeding bed after tumor enucleation with potency of 50W in spray mode offer an excellent hemostasis.
The use of this new technique extrapolated from liver surgery [13,14] was satisfactory.
Its reproduction in retroperitoneoscopy showed some limitations due to the large amount of smoke released by the process in small space, what can be more laborious and require more aspiration to achieve better visualization. Therefore, laparoscopic pure or robotic assisted, transperitoneal route is the most indicated if greased lidocaine is used.
The aim of applying this technique is to make feasible enucleation of small and low complexity tumors without clamping the renal hilum and sutures with low cost and safety in its execution.
In this preliminary experience we observe that greased lidocaine gel after surgery for small renal mass promote:
1) Good efficacy in hemostasis after partial nephrectomy or enucleation by RENAL Score tumors of low complexity;
2) Safety in terms of the depth of thermal injury seen in swine models;
3) Low cost;
4) Absence of artifacts in CT scan evaluation.
- Chen DYT, Uzzo RG. Optimal management of localized renal cell carcinoma: surgery, ablation, or active surveillance. J Natl Compr Canc Netw. 2009; 7: 635–642. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19555585
- Kunath F, Schmidt S, Krabbe L-M, Miernik A, Dahm P, et al. Partial nephrectomy versus radical nephrectomy for clinical localised renal masses. Cochrane Database Syst Rev. 2017; 5: CD012045. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28485814
- Russo P. End stage and chronic kidney disease: associations with renal cancer. Front Oncol. 2012; 2: 28. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22649783
- Kara O, Maurice MJ, Malkoc E, Ramirez D, Nelson RJ, et al. Comparison of robot-assisted and open partial nephrectomy for completely endophytic renal tumours: a single centre experience. BJU Int. 2016; 118: 946–951. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27477777
- Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009; 182: 844–853. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19616235
- Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010; 14: R52. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20370902
- Metro MJ, McAninch JW. Surgical exploration of the injured kidney: current indications and techniques. Int Braz J Urol. 2003; 29: 98–105. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15745491
- Rosales A, Salvador J, De Graeve N, Angerri O, Villavicencio H. Clamping of the renal artery in laparoscopic partial nephrectomy: an old device for a new technique. Eur Urol. 2005; 47: 98–101. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15582256
- Abarzua-Cabezas FG, Sverrisson E, De La Cruz R, Spiess PE, Haddock P, et al. Oncological and functional outcomes of salvage renal surgery following failed primary intervention for renal cell carcinoma. Int Braz J Urol. 2015; 41: 147–154. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25928521
- Tanagho YS, Bhayani SB, Figenshau RS. Robot-assisted partial nephrectomy in contemporary practice. Front Oncol. 2012; 2: 213. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23336101
- Larcher A, Sun M, Dell’Oglio P, Trudeau V, Boehm K, et al. Mortality, morbidity and healthcare expenditures after local tumour ablation or partial nephrectomy for T1A kidney cancer. Eur J Surg Oncol. 2017; 43: 815–822. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27692535
- Tsui KH, van Ophoven A, Shvarts O, Belldegrun A. Nephron-sparing surgery for renal cell carcinoma. Rev Urol. 1999; 1: 216–225. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16985800
- de Matos Filho AS, Petroianu A, Alberti LR, Vidigal PVT, dos Reis DCF, de Souza DM. Liver hemostasis using a dry electrocautery or greased with lidocaine or neomycin or glycerin or vaseline, in rabbit. Rev Col Bras Cir. 2009; 36: 442–448. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20069158
- Petroianu A. Hemostasis of the liver, spleen, and bone achieved by electrocautery greased with lidocaine gel. Surg Today. 2011; 41: 300–302. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21264774