More Information
Submitted: March 28, 2022 | Approved: May 25, 2022 | Published: May 26, 2022
How to cite this article: Almadhoni M, Baggas ML. Pituitary gland metastasis from breast cancer: case report. Arch Cancer Sci Ther. 2022; 6: 001-003.
DOI: 10.29328/journal.acst.1001025
Copyright License: © 2022 Almadhoni M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Pituitary gland metastasis from breast cancer: case report
Mohamed Almadhoni1 and Mohamed Ali Baggas2*
1Mediterranean Clinic, Tripoli, Libya
2Department of Surgery, University of Tripoli, Libya
*Address for Correspondence: Mohamed Ali Baggas, Department of Surgery, University of Tripoli, Libya, Email: [email protected]; [email protected]
Cancer metastasis to the pituitary gland is rare, but in most cases, it originates from a late-stage breast tumor or lung cancer. The most common symptoms of metastasis to the pituitary gland are diabetes insipidus and visual disturbance. The common site of metastasis is the posterior portion of the pituitary gland because it is highly vascularized. Metastases to this site represent 1% of all tumors [1]. Metastasis to the pituitary gland is difficult to diagnose by hormonal analysis and magnetic resonance imaging of the brain and requires biopsy for confirmation [2].
The recent increase in survival of cancer patients and the advancement in diagnostic techniques have resulted in a more frequent diagnosis of breast and lung cancer metastasis to the pituitary gland, which has a poor prognosis [3,4].
A 43-years old woman with right breast carcinoma since 2015 and with lymph node and bone metastases that developed after treatment with chemotherapy, hormones, radiotherapy, and surgical ablation of the ovaries presented at the Tripoli University Hospital in February 2017. She presented with a history of repeated attacks of vomiting for two months and a history of generalized fatigability and postural dizziness that worsened in the preceding two weeks, as well as marked weight and appetite loss, and diminution of vision. On examination, her blood pressure was 90/60 mmHg, and her pulse rate was 62 bpm. There was no lymphadenopathy or organomegaly. Initially, a pituitary macroadenoma was suspected because of visual disturbances.
Laboratory investigations
The patient’s laboratory results on admission revealed electrolyte disturbances in the form of hypernatremia and hypokalemia with low pituitary hormones and high tumor markers and LDH. Her urine output was exceeding 4 liters/24h (Tables 1,2). She was managed with intravenous fluids, hydrocortisone, and antibiotics, and the following workup was carried out.
| Table 1: Laboratory data of the patient. | ||
| Analyte | Value | Normal range |
| General hematology and serum chemistry | ||
| Leukocytes (109/L,) | 4.7 | |
| Hemoglobin (g/dl) | 9.9 | |
| Platelets (109/L,) | 173 | |
| Random blood sugar (mg/dl) | 91 | |
| Urea (mg/dl) | 16 | |
| Creatinine (mg/dl)) | 0.8 | |
| Na+ (mg/dl) | 148 | |
| K+ (mg/dl) | 3.2 | |
| Cl− (mg/dl) | 108 | |
| Total bilirubin (mg/dl) | 0.35 | |
| LDH (UI/l) | 1706 | |
| ALP (UI/l) | 505 | |
| AST (UI/l) | 81 | |
| ALT (UI/l) | 32 | |
| Serum magnesium (mg/dl) | 3.1 | |
| Basal cortisone (nmol/l) | 17.4 | 171-536 |
| Pituitary hormones | ||
| Prolactin (ng/ml) | 0.441 | 4.79-23.3 |
| TSH (mIU/L) | 0.012 | 0.27-4.20 |
| T4 (nmol/l) | 66.3 | 66.0-81.0 |
| T3 (nmol/l) | 1.73 | 1.3-3.1 |
| FSH (IU/L) | < 1.0 | 4.5-21.5 |
| LH (IU/L) | < 1.0 | 5-25 |
| Tumor markers | ||
| CA 15-3 (U/ml) | 1079.9 | < 30 |
| CA 125 (U/ml) | 89.9 | < 46 |
| Table 2: Post-operative laboratory data 10 days after admission. | ||||||||||
| Erythrocytes x106/µl | 10.6 | 16.9 | 15.8 | 12.7 | 8.9 | 7.5 | 10.2 | 7.1 | 10.8 | 5.2 |
| Hemoglobin (g/dl) | 8.1 | 12.5 | 10.7 | 11.6 | 12 | 12.4 | 12.2 | 11.5 | 10.2 | 9.5 |
| Platelets (109/L) | 227 | 206 | 180 | 201 | 173 | 173 | 182 | 155 | 191 | 212 |
| Na+ (mg/dl) | 146 | 153 | 156 | 169 | 166 | 164 | 152 | 158 | 156 | 155 |
| K+ (mg/dl) | 3.3 | 3.3 | 2.3 | 3.4 | 3 | 3.4 | 3.8 | 4.5 | 3.8 | 4.2 |
| Cl̶̶ (mg/dl) | 117 | 123 | 128 | 136 | 133 | 132 | 129 | 128 | 122 | 120 |
| Blood sugar profile (mg/dl) | 149 | 96 | 114 | 110 | 146 | 129 | 150 | 148 | 174 | 111 |
| Creatinine (mg/dl) | 2.1 | 2.2 | 2.9 | 2.4 | 1.9 | 0.2 | 1.6 | 1.3 | 1.1 | 1.4 |
| Urea (mg/dl) | 26 | 28 | 37 | 54 | 71 | 77 | 81 | 77 | 73 | 75 |
| Input (ml) | 3800 | 3850 | 4800 | 1900 | 4950 | 3750 | 1910 | 3550 | 3950 | 3200 |
| Output (ml) | 3700 | 3000 | 3800 | 2500 | 4130 | 2200 | 2600 | 4000 | 3100 | 3050 |
In an ultrasound of the abdomen, the liver appeared normal and no focal lesion or sharp edge was observed. Gall bladder, common bile duct, and intrahepatic ducts were normal. Spleen, pancreas, and both kidneys were also normal, and no ascites or para-aortic lymphadenopathy was detected.
In computerized scanning (Siemens Somatom Emotion 16 CT Scanner), the chest, abdomen pelvis, lungs, liver, gall bladder, spleen, pancreas, kidneys, adrenals, and urinary bladder were normal. Multiple bone lesions were present at the dorsolumbar spine and pelvis, and enlarged axillary lymphadenopathy was suggestive of metastasis.
CT of the brain showed a small contrast-enhanced suprasellar lesion requiring magnetic resonance imaging (MRI) for evaluation of the pituitary. MRI of the brain and pituitary gland revealed an enlarged pituitary gland with a small infudibular enhanced mass (8 x 8 mm) to be followed up and the results correlated with laboratory findings.
The patient was diagnosed as having pituitary adenoma with panhypopituitarism and discharged on hydrocortisone tablets (30 mg in the morning and 20 mg in the evening), as well as one 50 mg tablet of L-thyroxine daily.
She was followed up in the endocrinology and oncology outpatient departments. Over the subsequent months, the patient progressively deteriorated. By the second of January 2018, she developed bilateral loss of vision and frequent attacks of hypotension and hypoglycemia.
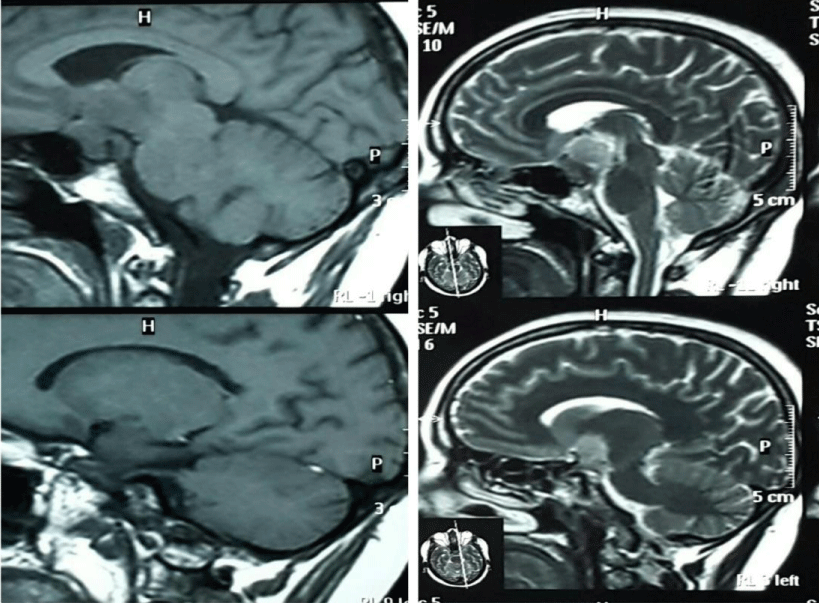
MRI of the brain and pituitary gland showed increased contrast enhancement of the sellar and suprasellar mass (28 x 18 mm) with compression and displacement of the optic chiasma and tract reaching to the floor of the third ventricle and encasing the cavernous sinus on both sides. There was no evidence of intralesional hemorrhage (Figure 1).
Figure 1: MRI. T1 T2 sequences with contrast, 3,5 mm thickness showed evidence of large well defined sellar and suprasellar mass lesion 18 mm by 28 mm, hemogenously enhanced post IV contrast injection causing compression on optic chiasma reaching to 3th ventricle floor and encasing cavernous sinus on both side.
Surgery
The patient was admitted to the intensive care unit, and intravenous saline and dexamethasone were started. On the third of January 2018, the patient underwent pterional craniotomy and decompression, and a biopsy was taken. The pituitary tumor was resected by trans-cranial surgery. Post-operatively, the patient was kept in the intensive care unit for close observation. On the second day, the vision and consciousness levels improved. The patient was monitored closely for diabetes insipidus symptoms and managed with intravenous dexamethasone, low-strength saline, antibiotics, and routine postoperative care. Transient diabetes insipidus occurred but resolved without desmopressin. A follow-up CT scan of the brain showed a small subarachnoid hemorrhage. Brain MRI demonstrated gross total resection of the tumor.
Histopathological examination of the resected mass showed a poorly differentiated adenocarcinoma with signet ring features compatible with metastasis.
Outcomes
Postoperative follow-up continued for two weeks, and subsequent clinical imaging did not show local recurrence of the primary malignancy. She was started on desmopressin nasal spray and steroids. Her blood sugar level improved, with no more attacks of hypoglycemia, and her urine output stabilized at 3 liters. She continued to be followed up by an endocrinologist and an oncologist.
Mass lesions that can cause hypopituitarism include pituitary adenomas, cysts, lymphocytic hypophysitis, and metastatic cancer. Most reported cases of metastasis to the pituitary show that the primary tumor was in the breast or lung. Moreover, diabetes insipidus occurs in about 70% of the cases, and hypopituitarism occurs in 15% of symptomatic cases [5], It’s known that the Blood-Brain Barrier has an important role in brain metastasis formation, it forms a tight barrier protecting the central nervous system from cancer cells entrance, but the absence of the blood-brain barrier in the posterior pituitary region increase the chance of metastatic cells spread that may travel through the hypothalamus-hypophyseal portal circulation system, The most route for metastasis in posterior pituitary lobe by hematogenous spread through the inferior hypophyseal artery [6].
Any mass lesion in the sella can cause temporary or permanent damage by exerting pressure on pituitary cells. Symptoms occur in about 7% of patients with pituitary metastasis and commonly diabetes insipidus, occurring in about 50% – 70% of patients. The most common clinical symptoms are headache, fatigue, polyuria, visual loss [7,8], anterior pituitary dysfunction, visual field defects, retro-orbital pain, and ophthalmoplegia. The prognosis is poor and survival in one series averaged three to six months [9].
Patients may experience nausea, vomiting, fatigue, and weight loss. Therefore, it is not surprising that pan-hypopituitarism can be easily attributed to the side effects of chemotherapy received for the primary malignancy [10].
Pan-hypopituitarism and pituitary metastasis are likely underdiagnosed. However, when diabetes insipidus results in polyuria and polydipsia becomes generally a frank, the symptom makes the patient seek medical advice. Diagnosis of pituitary metastasis is difficult because up to 16% of such patients with overt malignancy may also have pituitary adenoma [11].
Because the clinical and radiological manifestations of malignant metastasis to the pituitary gland may mimic benign tumors, the location of pituitary involvement in imaging provides a higher possibility of differentiation between malignant and benign cancer. Most registered cases of metastasis to the pituitary were to the posterior lobe, which was attributed to its richer blood supply compared to the anterior lobe [12].
Treatment of metastasis to the pituitary consists of transcranial and transsphenoidal surgeries and treatment of the severe symptoms of diabetes insipidus and visual disturbance [13].
The trans-cranial approach can provide a definitive diagnosis by obtaining a biopsy of the sellar lesion, which requires an experienced neurosurgical team. It has been reported that while surgery provides histopathological confirmation of the disease, it does not increase the mean survival time, but it does improve the quality of life. Therefore, surgery should be performed on patients who have experienced a consistent reduction in the quality of their life or to decompress the optic nerves and chiasma [14].
The patient with metastatic cancer to the brain can present acutely with vomiting and hypotension, which are commonly attributed to chemotherapy side effects or poor nutrition. Physicians can forget to consider hypopituitarism caused by metastasis as a cause. This could lead to incorrect diagnosis and management and put the patient’s life at risk. It is essential to consider hypopituitarism in any patient with metastasis to the brain presenting with hypotension and electrolyte disturbance.
- Poursadegh Fard M, Borhani Haghighi A, Bagheri MH. Breast cancer metastasis to pituitary infandibulum. Iran J Med Sci. 2011 Jun;36(2):141-4. PMID: 23358184; PMCID: PMC3556747.
- Rajput R, Bhansali A, Dutta P, Gupta SK, Radotra BD, Bhadada S. Pituitary metastasis masquerading as non-functioning pituitary adenoma in a woman with adenocarcinoma lung. Pituitary. 2006;9(2):155-7. doi: 10.1007/s11102-006-8326-0. PMID: 16832588.
- Kurkjian C, Armor JF, Kamble R, Ozer H, Kharfan-Dabaja MA. Symptomatic metastases to the pituitary infundibulum resulting from primary breast cancer. Int J Clin Oncol. 2005 Jun;10(3):191-4. doi: 10.1007/s10147-004-0458-5. PMID: 15990968.
- Kovacs K. Metastatic cancer of the pituitary gland. Oncology. 1973;27(6):533-42. doi: 10.1159/000224763. PMID: 4355105.
- Peppa M, Papaxoinis G, Xiros N, Raptis SA, Economopoulos T, Hadjidakis D. Panhypopituitarism due to metastases to the hypothalamus and the pituitary resulting from primary breast cancer: a case report and review of the literature. Clin Breast Cancer. 2009 Nov;9(4):E4-7. doi: 10.3816/CBC.2009.n.047. PMID: 19933072.
- Wilhelm I, Molnár J, Fazakas C, Haskó J, Krizbai IA. Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci. 2013 Jan 11;14(1):1383-411. doi: 10.3390/ijms14011383. PMID: 23344048; PMCID: PMC3565326.
- Kim YH, Lee BJ, Lee KJ, Cho JH. A case of pituitary metastasis from breast cancer that presented as left visual disturbance. J Korean Neurosurg Soc. 2012 Feb;51(2):94-7. doi: 10.3340/jkns.2012.51.2.94. Epub 2012 Feb 29. PMID: 22500201; PMCID: PMC3322215.
- Kurkjian C, Armor JF, Kamble R, Ozer H, Kharfan-Dabaja MA. Symptomatic metastases to the pituitary infundibulum resulting from primary breast cancer. Int J Clin Oncol. 2005 Jun;10(3):191-4. doi: 10.1007/s10147-004-0458-5. PMID: 15990968.
- Altay T, Krisht KM, Couldwell WT. Sellar and parasellar metastatic tumors. Int J Surg Oncol. 2012;2012:647256. doi: 10.1155/2012/647256. Epub 2011 Oct 13. PMID: 22312541; PMCID: PMC3263702.
- Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, Thalassinos NC. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004 Feb;89(2):574-80. doi: 10.1210/jc.2003-030395. PMID: 14764764.
- Fortunati N, Felicetti F, Donadio M, Grossi E, Michelon F, Ritorto G, Arvat E, Brignardello E. Pituitary lesions in breast cancer patients: A report of three cases. Oncol Lett. 2015 Jun;9(6):2762-2766. doi: 10.3892/ol.2015.3149. Epub 2015 Apr 24. PMID: 26137142; PMCID: PMC4473682.
- Ozkan C, Arslan C, Kilic MK, Erman M, Altundag K. Pituitary Gland Metastasis of Breast Cancer: A Case Report. Int J Hematol Oncol. 2011;29(4): 042-045.
- Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, Thalassinos NC. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004 Feb;89(2):574-80. doi: 10.1210/jc.2003-030395. PMID: 14764764.
- Fassett DR, Couldwell WT. Metastases to the pituitary gland. Neurosurg Focus. 2004 Apr 15;16(4):E8. PMID: 15191337.