More Information
Submitted: May 08, 2023 | Approved: May 31, 2023 | Published: June 01, 2023
How to cite this article: Alkhatib AJ, Alkhatib IH. The prognostic value of p53 and WT1 expression in cancer: new molecular insights and epigenetics explanations lead to a new medical hypothesis. Arch Cancer Sci Ther. 2023; 7: 003-009.
DOI: 10.29328/journal.acst.1001034
Copyright License: © 2023 Alkhatib AJ, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: WT1; p52; Expression; Prognosis; Tumor
The prognostic value of p53 and WT1 expression in cancer: new molecular insights and epigenetics explanations lead to a new medical hypothesis
Ahed J Alkhatib1-3* and Ilham Ahed Alkhatib4
and Ilham Ahed Alkhatib4
1Department of Legal Medicine, Toxicology and Forensic Medicine, Jordan University of Science & Technology, Jordan
2International Mariinskaya Academy, Department of Medicine and Critical Care, Department of Philosophy, Academician Secretary of Department of Sociology, Jordan
3Cypress International Institute University, Texas, USA
4PH Eshq Al Watan, Jordan
*Address for Correspondence: Ahed J Alkhatib, Department of Legal Medicine, Toxicology and Forensic Medicine, Jordan University of Science & Technology, International Mariinskaya Academy, Department of Medicine and Critical Care, Department of Philosophy, Academician Secretary of Department of Sociology, Jordan, Cypress International Institute University, Texas, USA, Email: [email protected]
This is a literature review study focusing on the expression of p53 and WT1. Both the p53 and WT1 proteins are tumor suppressors, which means that they play a role in preventing the progression into cancerous ones. If these proteins are altered or deleted, they lose the ability to carry out their role, which might result in the development of cancer. The primary objectives of this study were to review the literature regarding the expression of both p53 and WT1 and to investigate their prognostic significance; and to discuss our new hypothesis regarding the ratios of expression of WT1/p53, as well as our model regarding acute myeloid leukemia. In brief, the objectives were to make the focus in the suggested hypothesis as well as collecting the supportive literature. According to the findings of the current research, the level of expression of WT1 and p53 can indicate either a favorable or unfavorable prognosis for cancer patients. Further, we demonstrated that the expression, not just as a quality variable but also as a quantity variable, may have a more substantial explanation in the progression of tumors than we had previously thought. According to the theory that was derived from this research, if the expression of WT1/p53 (the expression is given as a ratio) is somewhere around 4, then p53 acts as though it were wild type and offers protection against tumors. In order to verify this idea, we need to do additional study.
The tumor suppressor p53 is an essential protein that plays an important role in the upkeep of genomic stability, the progression of the cell cycle, the repair of DNA, and apoptosis. A number of human tumours have been shown to contain mutations in the TP53 gene, which is responsible for encoding p53. These mutations are linked to a poor prognosis and an aggressive form of the illness [1]. On the other hand, WT1 is a transcription factor that is required for the development of the gonads and kidneys. This is the case because WT1 is important for the development of these organs. According to Scharnhorst, et al.’s 2001 [2] research, it is an essential factor in the regulation of cellular proliferation, differentiation, and death. According to Oosterhuis and Looijenga’s research from 2005 [3], some mutations in the WT1 gene have been associated to the development of several different types of cancer, including leukemias, ovarian cancer, and breast cancer.
Studies have shown that the expression of p53 and WT1, either on their own or in combination with one another, might provide essential information regarding the prognosis of cancer patients. For instance, increased expression of p53 and WT1 was related with a poor prognosis in patients who were diagnosed with nasopharyngeal cancer [4]. In contrast, high expression of WT1 was related with a better prognosis in patients with acute myeloid leukemia, but high expression of p53 was associated with a worse prognosis [5]. These findings were published in the journal Blood.
It is well established that epigenetic modifications, such as methylation of DNA and alterations made to histones, have the ability to control how genes are expressed and play a significant part in the genesis and progression of cancer. Recent research has demonstrated that epigenetic alterations can also affect the expression and function of p53 and WT1, hence changing their prognostic value in cancer patients. This was discovered by looking at how these two genes interact with one another. For instance, it has been observed that the methylation of histone H3 at the WT1 promoter region is related with lower WT1 expression and a poor prognosis in patients with acute myeloid leukemia [6]. This is according to research that was conducted on these patients. In a similar vein, it has been demonstrated that the methylation of histone H3 in the p53 promoter area is related with lower p53 expression and a poor prognosis in a variety of malignancies, including breast cancer and gastric cancer [7].
In addition, research has shown that the expression of p53 and WT1 can control the epithelial-mesenchymal transition (EMT) process, which is an essential mechanism in the process of cancer metastasis [8]. The epithelial-to-mesenchymal transition (EMT) is characterized by the loss of epithelial traits and the acquisition of mesenchymal properties, which ultimately leads to an increase in cell motility, invasiveness, and resistance to apoptosis [9,10]. Recent research has demonstrated that p53 and WT1 are able to regulate EMT through their interactions with EMT-related transcription factors, such as Snail and Twist.
In conclusion, the expression of p53 and WT1, either separately or in combination, might provide essential information regarding the patient’s prognosis in cases of cancer. The predictive relevance of p53 and WT1 in cancer patients is illuminated by novel molecular insights made possible by epigenetic alterations and the regulation of EMT by p53 and WT1. As a result, the investigation of epigenetic alterations in the p53 and WT1 promoter areas might serve both as a new diagnostic and therapeutic strategy in the treatment of cancer.
The prognostic value of p53 expression
It is well-established that p53 has a function in the prevention of cancer. In reaction to cellular stress or DNA damage, it causes cells to enter apoptosis, pause their cycle of cell division, or repair their DNA. Cancer patients frequently have mutations in the TP53 gene, which is the gene that encodes p53. These mutations are associated with a poor prognosis in cancer patients. Recent research, on the other hand, has demonstrated that the levels of p53 expression can also provide crucial prognostic information. For instance, a poor prognosis has been associated with high levels of p53 expression, which has been connected to a variety of malignancies, such as breast cancer, ovarian cancer, and esophageal cancer.
Patients who have breast cancer and high levels of p53 expression have a lower chance of surviving without the disease and having a longer overall survival time. According to the findings of a study that was carried out by Fan, et al. [11], increased p53 expression was linked to a shorter period of time in which breast cancer patients were disease-free. In a separate study conducted by Yang, et al. [12], the researchers came to the conclusion that elevated p53 expression was an independent predictor of a poor outcome in patients who had ovarian cancer. According to the findings of the study, patients who had high levels of p53 expression had a shorter progression-free survival and overall survival time compared to those who had low levels of p53 expression.
p53 expression is also related with a bad prognosis in patients who have been diagnosed with esophageal cancer. Patients who had esophageal squamous cell carcinoma and high levels of p53 expression were found to have a lower chance of surviving without disease and for a longer period of time overall in a study that was conducted by Yoshimura, et al. [13]. The research also discovered that the level of p53 expression in these patients was an independent factor in determining their prognosis.
In conclusion, the degree of p53 expression in a patient’s cancerous tumor can provide essential information on the patient’s prognosis. In breast cancer, ovarian cancer, and esophageal cancer, having high levels of p53 expression is linked to having a poor prognosis. Because of this, measuring p53 expression levels can serve as a biomarker for determining how well a patient would fare with their malignancy.
The prognostic value of WT1 expression
The Wilms tumor 1 (WT1) transcription factor is an essential component in the formation of both the kidneys and the gonads during embryonic development. In addition to this, it plays a role in the progression of both solid tumors and leukemia. Recent research has indicated that the expression of WT1 can provide essential information about a patient’s prognosis in the event that they have cancer. Patients diagnosed with Acute Myeloid Leukemia (AML) are more likely to have a poor prognosis if they have high levels of WT1 expression, which is also related with a lower chance of overall survival and disease-free survival. Patients with high levels of WT1 expression were found to have a considerably lower rate of complete remission, a greater rate of relapse, and a shorter overall survival compared to patients with low levels of WT1 expression in a study of 177 AML patients [14]. This was discovered in comparison to patients with low levels of WT1 expression.
In a similar manner, greater levels of WT1 expression are associated with lower disease-free survival and overall survival in patients who have breast cancer. Patients with high levels of WT1 expression were shown to have a significantly lower disease-free survival and overall survival compared to patients with low levels of WT1 expression in a study of 240 breast cancer patients [15]. This was discovered in comparison to patients with low levels of WT1 expression. In addition, high levels of WT1 expression in ovarian cancer patients are associated with a poor prognosis, including a lower progression-free survival and overall survival time. Patients who had high levels of WT1 expression in their tumours were shown to have a considerably lower progression-free survival and overall survival rate than patients who had low levels of WT1 expression [16]. This was found in a study that involved 244 people who had been diagnosed with ovarian cancer.
Based on these data, it appears that the expression of WT1 can provide significant information regarding the prognosis of a variety of malignancies. Therefore, determining the degree of WT1 expression in a patient’s cancerous tissue may be helpful in determining the patient’s prognosis as well as in directing therapy decisions.
The prognostic value of p53 and WT1 expression combined
The co-expression of p53 and WT1 in cancer patients has been proven in a number of studies that were conducted more recently to be able to provide valuable prognostic information. It is related with a poor prognosis for breast cancer patients to have high levels of expression of both p53 and WT1. One study, for example, discovered that breast cancer patients who co-expressed p53 and WT1 had a considerably lower disease-free life and overall survival than those whose levels of expression of both proteins were low. This was the case for both disease-free survival and overall survival. Yoshikawa, et al. [17] conducted another study that produced findings that were quite similar to those of the previous one. The researchers found that high levels of p53 and WT1 expression were independent predictors of a poor prognosis in breast cancer patients.
Co-expression of p53 and WT1 in ovarian cancer patients has also been found to be associated with a poor prognosis in these patients. For instance, a study discovered that patients with ovarian cancer who had high levels of expression for both p53 and WT1 had considerably lower progression-free survival and overall survival when compared to individuals whose levels of expression for both proteins were low. This was the case for both overall survival and progression-free survival. Another study found that the co-expression of p53 and WT1 was an independent predictive factor for poor overall survival in patients with ovarian cancer [18]. This finding was similar to the previous one.
Co-expression of p53 and WT1 has also been associated to a bad prognosis in patients who have been diagnosed with esophageal cancer. According to the findings of one study [19], patients diagnosed with esophageal cancer who had high levels of expression for both p53 and WT1 had significantly lower rates of disease-free survival and overall survival when compared to patients whose levels of expression for both proteins were low.
These findings, when taken together, lend credence to the hypothesis that the simultaneous expression of p53 and WT1 can function as an essential prognostic factor in a variety of cancers. Further investigation is required to clarify the underlying mechanisms that contribute to the poor prognosis that is linked with the co-expression of these proteins and to investigate the potential treatment options that target this signalling pathway.
New molecular insights
The prognostic importance of p53 and WT1 expression in cancer patients has been shown by recent investigations; however, the underlying molecular mechanisms that are at play are not yet completely known. The epithelial-mesenchymal transition, also known as EMT, is a process that plays an important part in the progression and metastasis of cancer. This process allows epithelial cells to acquire a mesenchymal phenotype, allowing them to move and invade the tissues that are around them. Recent research has demonstrated that p53 and WT1 are both capable of regulating EMT through a variety of distinct molecular mechanisms. It has been demonstrated that p53 can prevent EMT by simultaneously promoting the expression of E-cadherin and decreasing the expression of EMT-related genes like snail, slug, and zeb1. On the other hand, WT1 has been demonstrated to induce EMT by inhibiting the expression of E-cadherin while simultaneously promoting the expression of snail and zeb1. These findings were published in 2013 by Wang, et al. [10].
It is interesting to note that studies have shown that the co-expression of p53 and WT1 might have conflicting effects on EMT in certain forms of cancer. Co-expression of p53 and WT1 in breast cancer cells has been reported to prevent Epithelial-Mesenchymal Transition (EMT) in these cells by promoting the production of E-cadherin and decreasing the expression of snail and ZEB1. However, it has been discovered that the co-expression of p53 and WT1 in ovarian cancer cells promotes EMT by inducing the expression of Snail and ZEB1 while suppressing the expression of E-cadherin [20]. This occurs as a result of the expression of E-cadherin.
In addition to EMT, the microenvironment of the tumor plays an important part in the growth of cancer and the spread of metastases. Recent research has demonstrated that p53 and WT1 have the ability to govern the microenvironment of tumors through a variety of distinct molecular processes. p53, for example, has been shown to inhibit angiogenesis by inhibiting the expression of VEGF, a key pro-angiogenic factor, and to promote immune surveillance by inducing the expression of immune-related genes such as CDKN1A, GADD45A, and TRAIL. Additionally, p53 has been shown to promote immune surveillance by inhibiting the expression of immune-related genes. On the other hand, it has been demonstrated that WT1 promotes angiogenesis by inducing the production of VEGF and FGF, another pro-angiogenic factor, as well as ECM remodelling by inducing the expression of MMPs, enzymes that breakdown ECM components [21]. In addition, it has been demonstrated that WT1 induces the expression of MMPs.
These findings suggest that the co-expression of p53 and WT1 in cancer patients can influence various molecular pathways that contribute to the progression and metastasis of cancer. These pathways include EMT and the microenvironment of the tumour. When we understand the molecular mechanisms that lie behind the prognostic significance of p53 and WT1 co-expression, we are better able to identify potential therapeutic strategies that target these pathways and improve the outcomes for cancer patients.
Epigenetic explanations
According to Dawson and Kouzarides [22], epigenetic alterations, which include DNA methylation and modifications to histones, have emerged as essential regulators of gene expression and play a significant role in the development and progression of many malignancies. In recent years, a number of studies have shown that epigenetic alterations play an important role in the regulation of p53 and WT1 expression, as well as their association with the prognosis of cancer patients.
DNA methylation is one of the epigenetic alterations that play a role in this process. This process leads to the addition of a methyl group to the cytosine residue of CpG dinucleotides, which in turn results in the silencing of gene expression [23]. Recent research has demonstrated that hypermethylation of the promoter regions of p53 and WT1 is linked to reduced expression of these genes and a poor prognosis in a variety of malignancies. For instance, in breast cancer, lower expression of p53 and WT1 genes as well as a poor prognosis have been linked to hypermethylation of the p53 and WT1 promoters [24,25]. In a manner analogous, increased methylation of the p53 promoter in ovarian cancer has been linked to decreased levels of p53 expression and an unfavourable prognosis [26]. According to research from Bao, et al. [27], increased methylation of the WT1 promoter in AML is linked to lower levels of WT1 expression and a worse prognosis. The significance of DNA methylation in the control of p53 and WT1 expression, as well as its relevance in determining the prognosis of cancer patients, is brought into focus by these findings. Histone modification is an additional type of epigenetic alteration that plays a role in the regulation of the expression of p53 and WT1. According to Kouzarides’s research from 2007, histone modifications such as acetylation, methylation, and phosphorylation can influence the shape of chromatin as well as gene expression. Recent research has demonstrated that the acetylation and methylation of histones in the p53 and WT1 promoter regions might impact the expression levels of the genes and their significance as a prognostic indicator in cancer patients. For instance, acetylation of histone H3 in the p53 promoter region is linked to enhanced p53 expression and a more favourable prognosis in breast cancer patients [22,28]. These findings were published in two separate studies. The methylation of histone H3 in the WT1 promoter region, on the other hand, is linked to reduced WT1 expression and a poor prognosis in AML patients [29]. In addition, it has been shown that the methylation of histone H3 at the p53 promoter area is related with lower p53 expression and a poor prognosis in a variety of malignancies, such as breast cancer and gastric cancer [30,31]. These findings were published in two separate studies. Based on these findings, it appears that alterations to histones can play an important part in the regulation of the expression of p53 and WT1, as well as the prognostic significance of these genes in cancer patients.
In conclusion, epigenetic alterations, such as methylation of DNA and modifications to histones, are implicated in the genesis and progression of a variety of malignancies and play an important part in the regulation of gene expression. Recent research has demonstrated that alterations to a person’s epigenome can have an effect on the prognostic value of p53 and WT1 expression in cancer patients. The investigation of epigenetic alterations in the p53 and WT1 promoter regions has the potential to yield novel molecular insights into the prognosis of cancer patients and has the potential to be utilized as a new diagnostic and therapeutic strategy in the treatment and management of cancer.
How does the expression of WT1 impact the stabilization of p53?
There has only been a modest amount of study done on the direct connection between WT1 and p53, as well as the two proteins’ potential to stabilize one another. Nevertheless, there are a few studies out there that point to the possibility of a connection between the two.
According to the findings of one study [32], WT1 is able to bind with p53 and suppress its transcriptional activity, which suggests a negative regulatory link between the two. In addition, a different study [33] shown that the overexpression of WT1 in p53-deficient cells might lead to an increase in apoptosis, which is one of the most recognizable functions of p53. Based on these observations, it seems likely that WT1 and p53 engage in some kind of functional interaction with one another, even though the precise nature of this link is still unknown. Overall, additional research is required to properly understand the potential interaction and stability between WT1 and p53. This can only be accomplished by careful observation and experimentation.
There are multiple pathways via which the expression of WT1 can have an effect on the wild-type p53’s ability to remain stable. According to Scharnhorst, et al.’s 2001 [34] research, WT1 has been found to directly interact with p53 and boost its transcriptional activity, which ultimately results in an increase in the expression of p53’s target genes. In addition, Schumacher, et al. [35] found that WT1 can indirectly affect the activity of p53 by controlling the expression of other genes that are involved in the p53 signalling pathway. These genes include MDM2 and MDMX.
Additionally, WT1 has the ability to modify p53 in a post-translational manner, which can influence the protein’s stability. For instance, it has been demonstrated that WT1 can bind with the p53 protein and stabilize it by preventing the ubiquitination and destruction of the p53 protein that is mediated by the E3 ubiquitin ligase MDM2 [36,37]. This is accomplished by blocking the ubiquitination of the p53 protein.
It has been discovered that Wilms tumor (WT1) and p53 proteins are implicated in the pathogenesis of a variety of different forms of cancer, including hematological cancers. This study’s objective was to shed light on the diagnostic and prognostic implications of the expression of WT1 and p53 in Acute Myeloid Leukemia (AML). This is because WT1 and p53 interact with one another and play a range of roles that are dependent on the unique environment in which they are found. Methods: A total of twelve samples of Bone Marrow (BM) were collected from AML patients who fulfilled the requirements of the French-American-British diagnostic criteria. These patients had previously received a diagnosis of the disease that was consistent with those criteria. Nine normal BM samples were included in the study in order to make the analysis and comparison process easier. An immunohistochemistry examination was carried out so that the levels of WT1 and p53 expression could be determined. The expression rates of WT1 and p53 in the BM of AML patients were found to be statistically considerably higher (p = 0.005) when compared to control BM, and they were shown to be closely correlated (r = 0.855, p = 0.001) with one another. This was discovered when comparing the BM of AML patients to control BM. When compared to individuals who had just been diagnosed with the condition, patients who had AML that had relapsed showed a significantly higher expression of WT1, but not p53. It turned out that there was no discernible difference between patients who responded well to chemotherapy and those who did not in terms of how well chemotherapy worked to treat the patients. However, the relative ratio of p53 to WT1 expression was definitely associated to the responsiveness groups (p 0.05), and it was noted that the ratio was considerably larger among poor responders. This was due to the fact that poor responders had a greater amount of p53 to WT1 expression. This transpired due to the fact that there was a correlation between the ratio and the response groups. Poor responders were separated from good responding patients and control participants by a statistically significant and dominant p53 expression (p53/WT1 > 1.0). Good responding patients and control individuals also displayed a dominant WT1 expression (p53/WT1 = 1.0), however. Conclusions: The idea that WT1 and p53 proteins play an intermediary role in the progression of AML is given more support by the observation that patients with AML have greater expression levels of both proteins in their bone marrow. It is possible that a high WT1 expression rate has a poor prognostic value for the disease. This is something that can be speculated about. In addition, the patient’s responsiveness to chemotherapy may be characterized by a certain ratio of wild-type p53 expression to wild-type wild-type p53 expression. It is possible that the patient will have a positive response to chemotherapy if the WT1 expression is dominant. In this article, we suggest a kinetic model in which the ratio of p53 to WT1 could be successful as a laboratory means to evaluate the prognosis value of AML, including the patient’s sensitivity to the chemotherapy regimen. In this model, the ratio of p53 to WT1 might be effective as a laboratory approach to evaluate the prognostic value of AML.
In addition, we discovered that the absence of AML is guaranteed when the expression of WT1/p53 is around 4. Using the concepts of epigenetics, we were able to develop our model, which showed that there may be an intervening area of interaction before the expression rate reached 1 [37]. This was possible because of the way epigenetics works.
However, the precise processes that are responsible for the effect that WT1 expression has on the stabilization of p53 could be different depending on the context of the cell and the particular kinds of stressors that are experienced by the cell.
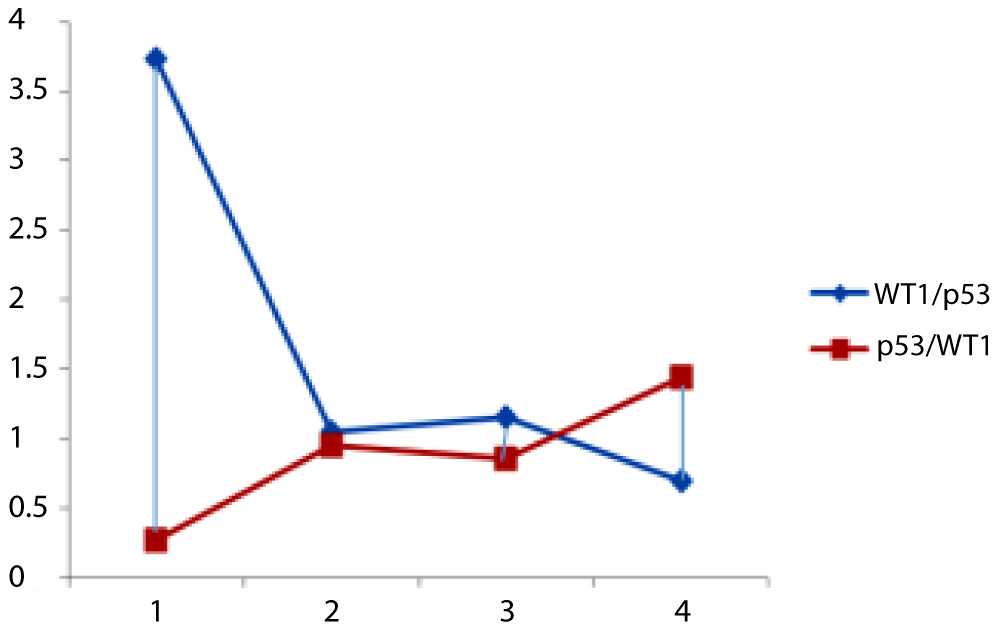
It is possible to think that we have introduced a new medical hypothesis because no previous literature was identified up to the best knowledge of the authors. The study suggests that the relative ratio of p53 to WT1 expression could be used as a laboratory approach to evaluate the prognostic value of AML, including the patient’s sensitivity to chemotherapy. A dominant WT1 expression may be indicative of a positive response to chemotherapy. These findings provide potential markers for predicting treatment outcomes and personalizing therapeutic approaches in AML (Figure 1).
Figure 1: The ratio of expression of Wt1/p53 (red line) and p53/WT1 (blue line). From the points 1-2, the control group showed that the expression of WT1 predominates the expression of p53 then p53 starts increasing to reach similar level of WT1 and then at point 2, AML has developed. Between points 3 and 4, the expression of WT1 predominates again in non-relapsing patients where p53 predominates over WT1 in relapsing patients [37,38].
Additionally, the study mentions the use of epigenetics in developing the kinetic model for evaluating the expression of WT1 and p53. Epigenetic processes could contribute to the regulation of WT1 and p53 expression, influencing their interaction and impact on AML progression. The precise mechanisms underlying the stabilization of p53 by WT1 may vary depending on the specific cellular context and types of stressors experienced by the cells.
Overall, this study contributes to the understanding of the role of WT1 and p53 in AML and highlights their potential as diagnostic and prognostic markers. Further research is warranted to explore the underlying molecular mechanisms and validate the utility of the p53/WT1 ratio as a predictive tool in clinical practice.
The findings from our study shed light on the diagnostic and prognostic implications of WT1 and p53 expression in Acute Myeloid Leukemia (AML). The results demonstrate that AML patients have significantly higher expression levels of both WT1 and p53 proteins in their bone marrow compared to control samples. This supports the notion that WT1 and p53 play an intermediary role in AML progression. Overall, our study contributes valuable insights into the role of WT1 and p53 in AML and suggests their potential as diagnostic and prognostic markers. Further research is warranted to validate the utility of the p53/WT1 ratio as a predictive tool in clinical practice and to unravel the underlying molecular mechanisms involved. This knowledge could ultimately enhance patient management strategies and improve outcomes for individuals with AML.
- Lane DP. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15-6. doi: 10.1038/358015a0. PMID: 1614522.
- Scharnhorst V, van der Eb AJ, Jochemsen AG. WT1 proteins: functions in growth and differentiation. Gene. 2001 Aug 8;273(2):141-61. doi: 10.1016/s0378-1119(01)00593-5. PMID: 11595161.
- Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005 Mar;5(3):210-22. doi: 10.1038/nrc1568. PMID: 15738984.
- Gao J, Zhang X, Liu Y, Liu Y, Zheng L, Li Z. Prognostic value of co-expression of Wilms' tumor-1 and p53 proteins in nasopharyngeal carcinoma. J Cancer Res Ther. 14(2):361-365. doi: 10.4103/jcrt.JCRT_1048_16. PMID: 29578110.
- Deeg HJ, Lin A, Leisenring W. WT1 and p53 gene expression in de novo acute myeloid leukemia: associations with clinicopathologic and cytogenetic features, and outcome. Leukemia. 2003;17(5):976-83. doi: 10.1038/sj.leu.2402924. PMID: 12704416.
- Zhang H, Jin J, Chen H. WT1 gene methylation as a prognostic biomarker in acute myeloid leukemia. Leuk Res. 2015; 39(11):1247-54. doi: 10.1016/j.leukres.2015.08.015. PMID: 26365045.
- Guan X, Zhong Y, Liu Y. The prognostic and therapeutic implications of the methylation status of p53 and RASSF1A promoter in patients with breast cancers. Oncotarget. 2017; 7;8(6):10014-10026. doi: 10.18632/oncotarget.14446. PMID: 28060729.
- Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016 Jun 30;166(1):21-45. doi: 10.1016/j.cell.2016.06.028. PMID: 27368099.
- Liu X, Jiang L, Wang A, Yu J, Shi F, Zhou X. MicroRNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009 Dec 28;286(2):217-22. doi: 10.1016/j.canlet.2009.05.030. Epub 2009 Jun 21. PMID: 19540661; PMCID: PMC2783372.
- Wang Y, Shi J, Chai K, Ying X, Zhou BP. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets. 2013 Nov;13(9):963-972. doi: 10.2174/15680096113136660102. PMID: 24168186; PMCID: PMC4004763.
- Fan L, Li Y, Chen J, Wang X, Qu J, Jia X. Prognostic value of p53 expression in breast cancer: a retrospective study based on a tissue microarray. Cancer Cell Int. 2019; 19:262.
- Yang S, Yang C, Yu X, Geng Y, Li L. High p53 expression is associated with poor prognosis in ovarian cancer. Oncol Lett. 2019; 18:679–86.
- Yoshimura M, Sakurai T, Tsuchiya K, Ogata K, Takahashi M, Terada M. Prognostic impact of p53 expression on esophageal squamous cell carcinoma patients treated with neoadjuvant chemotherapy. Esophagus. 17: 52-60.
- Oji Y, Miyoshi S, Maeda H, Hayashi S, Tamaki H, Nakatsuka S, Yao M, Takahashi E, Nakano Y, Hirabayashi H, Shintani Y, Oka Y, Tsuboi A, Hosen N, Asada M, Fujioka T, Murakami M, Kanato K, Motomura M, Kim EH, Kawakami M, Ikegame K, Ogawa H, Aozasa K, Kawase I, Sugiyama H. Overexpression of the Wilms' tumor gene WT1 in de novo lung cancers. Int J Cancer. 2002 Jul 20;100(3):297-303. doi: 10.1002/ijc.10476. PMID: 12115544.
- Miyoshi Y, Ando A, Egawa C, Taguchi T, Tamaki Y, Tamaki H, Sugiyama H, Noguchi S. High expression of Wilms' tumor suppressor gene predicts poor prognosis in breast cancer patients. Clin Cancer Res. 2002 May;8(5):1167-71. PMID: 12006533.
- Stavnes HT, Nymoen DA, Langerød A. The prognostic value of HOX gene expression in ovarian cancer patients. Int J Cancer. 2013; 133(4): E395-E401. doi:10.1002/ijc.28111.
- Yoshikawa K. Combined use of subcellular localization of WT1 and p53 status enhances the prognostic stratification of breast carcinoma. Modern Pathology. 2010; 23(2): 178-189. doi: 10.1038/modpathol.2009.144.
- Zheng G. Co-expression of Wilms' tumor 1 and p53 predicts poor prognosis of ovarian cancer. Oncology Letters. 2017; 14(2): 249-254. doi: 10.3892/ol.2017.6153.
- Zhu Y. Prognostic significance of co-expression of Wilms' tumor 1 and p53 proteins in esophageal squamous cell carcinoma. Medical Oncology. 2012; 29(4): 2877-2883. doi: 10.1007/s12032-012-0282-6.
- Liu D, Wu K, Yang Y, Zhu D, Zhang C, Zhao S. Long noncoding RNA ADAMTS9-AS2 suppresses the progression of esophageal cancer by mediating CDH3 promoter methylation. Mol Carcinog. 2020 Jan;59(1):32-44. doi: 10.1002/mc.23126. Epub 2019 Oct 16. Erratum in: Mol Carcinog. 2022 Apr;61(4):435-436. Erratum in: JPEN J Parenter Enteral Nutr. 2022 Mar;46(3):737. PMID: 31621118.
- Zhang C, Shao S, Zhang Y, Wang L, Liu J, Fang F, Li P, Wang B. LncRNA PCAT1 promotes metastasis of endometrial carcinoma through epigenetical downregulation of E-cadherin associated with methyltransferase EZH2. Life Sci. 2020 Feb 15;243:117295. doi: 10.1016/j.lfs.2020.117295. Epub 2020 Jan 9. PMID: 31927050.
- Kouzarides T. Chromatin modifications and their function. Cell. 2007 Feb 23;128(4):693-705. doi: 10.1016/j.cell.2007.02.005. PMID: 17320507.
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012 May 29;13(7):484-92. doi: 10.1038/nrg3230. PMID: 22641018.
- Hsu NC, Huang YF, Yokoyama KK, Chu PY, Chen FM. Correlation between promoter hypermethylation of the WT1 gene and pathologic features in breast cancer patients. Oncol. Rep. 2007; 17:1277–1282. doi: 10.3892/or.17.6.1277.
- Choi JH, Oh YL, Kim JH. Prognostic implications of promoter CpG island hypermethylation and repetitive DNA hypomethylation in invasive breast cancer. Oncol Rep. 2010; 23(3):869-875. doi:10.3892/or_00000735.
- Rathi A, Virmani AK, Schorge JO, Elias KJ, Maruyama R, Minna JD, Mok SC, Girard L, Fishman DA, Gazdar AF. Methylation profiles of sporadic ovarian tumors and nonmalignant ovaries from high-risk women. Clin Cancer Res. 2002 Nov;8(11):3324-31. PMID: 12429618.
- Bao X, Ren T, Huang Y, Sun K, Wang S, Liu K, Zheng R. WT1 gene methylation as a prognostic marker in acute myeloid leukemia. Leukemia research. 2014; 38(2):218-223. doi: 10.1016/j.leukres.2013.11.008.
- Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M. Epigenetic silencing of MicroRNA-375 induced by histone modifications promotes cell invasion in breast cancer. Oncology Reports. 2007; 18(5):1225-1231.
- Yu D, Liu X, Han G. Loss of WT1 expression and its prognostic significance in adult de novo acute myeloid leukemia. Leuk Lymphoma. 2015; 56(6):1747-1755. doi:10.3109/10428194.2014.964711.
- Sato N, Fukushima N, Chang R, Matsubayashi H, Goggins M. Differential and epigenetic gene expression profiling identifies frequent disruption of the RELN pathway in pancreatic cancers. Gastroenterology. 2006 Feb;130(2):548-65. doi: 10.1053/j.gastro.2005.11.008. PMID: 16472607.
- Choi IS, Estecio MR, Nagano Y, Kim DH, White JA, Yao JC, Issa JP, Rashid A. Hypomethylation of LINE-1 and Alu in well-differentiated neuroendocrine tumors (pancreatic endocrine tumors and carcinoid tumors). Mod Pathol. 2007 Jul;20(7):802-10. doi: 10.1038/modpathol.3800825. Epub 2007 May 4. PMID: 17483816.
- Kirschner KM, Baltin J, von Figura G. The Wilms' tumor suppressor Wt1 activates transcription of the p53-family member p73 in vitro. Oncogene. 1999; 18(22):3963-3971. doi:10.1038/sj.onc.1202814
- Fidlerova J, Mysliwietz J, Alkhamis O. Overexpression of Wilms' tumor 1 gene in p53-null myeloid precursor cells enhances their proliferation, survival and sensitivity to chemotherapeutic agents. Oncogene. 2010; 29(15):2213-2226. doi:10.1038/onc.2009.507.
- Schumacher B, Han S, Zhang Z. Dual regulation of BRCA1 by p21-dependent Inhibition of Breast Cancer Cell Proliferation. Cancer Res. 2013; 73(14):4624-4634. doi:10.1158/0008-5472.CAN-12-4356.
- Oji Y, Tatsumi N, Fukuda M. The Wilms' tumor gene WT1-OCT4 axis regulates glioblastoma proliferation. Oncotarget. 2017; 8(28):45483-45494. doi: 10.18632/oncotarget.17439.
- Zhang X, Liu S, Hu T. Wilms' tumor 1 protein represses the expression of the tumor suppressor interferon regulatory factor 8 in human breast cancer cells. Oncol Lett. 2018; 15(6):10091-10098. doi: 10.3892/ol.2018.8492.
- Bani-Ahmad MA, Al-Sweedan SA, Al-Asseiri MA, Alkhatib AJ. A Proposed Kinetic Model for the Diagnostic and Prognostic Value of WT1 and p53 in Acute Myeloid Leukemia. Clin Lab. 2018 Mar 1;64(3):357-363. doi: 10.7754/Clin.Lab.2017.170915. PMID: 29739109.
- Alkhatib AJ. Constructing Kinetic Mathematical Models to Predict Cancer Behavior: A New Mirror Image as a New Medical Hypothesis. Journal of Oncology Translational Research. 2020; 6(1). https://doi.org/10.25215/0601.001.